Briefing Document: Titration Curve Generator - Open Educational Resources / Open Source Physics @ Singapore
1. Introduction:
This document analyzes a resource from Open Educational Resources / Open Source Physics @ Singapore, specifically focusing on a "Titration Curve Generator" created by Grace Leong. This tool is designed to help students understand and interpret titration curves, a fundamental concept in chemistry. The resource is targeted towards Junior College level students studying H2 Chemistry and includes interactive simulations to enhance learning. The key learning objectives are to understand the changes in pH during acid-base titrations and to explain these changes based on the strengths of the acids and bases involved.
2. Key Concepts and Objectives:
- Titration Definition: Titration is defined as "the slow addition of a solution with known concentration (called a titrant) to a known volume of another solution with unknown concentration until the end point." It's used to determine unknown substance amounts in various industries.
- Titration Curves: A titration curve is a graph of pH against the volume of titrant added. It depicts the changes in pH of a solution during titration, thus is a tool to understand and analyze chemical reactions.
- Learning Objectives: Students should be able to:
- Describe pH changes during acid-base titrations.
- Explain these changes based on the strengths of acids and bases involved.
- Read and interpret scientific graphs, using titration curves as a specific example.
- Prerequisite Knowledge: Students should have a foundation in:
- Mole concept and stoichiometry.
- Acid and base theories.
- Chemical equilibria.
- Differences between strong and weak acids (dissociation extent).
- Understanding and use of Ka, pKa, Kb, pKb, and Kw including the relationship Kw=KaKb.
- Calculating [H+] and pH for strong acids, weak acids, strong bases, and weak bases.
- Buffer solution principles and pH calculations.
3. Titration Curve Generator:
- Purpose: The generator is a simulation tool designed to create and explore different titration curves.
- Usage: The resource provides access to the simulation as well as instructions on how to use it. This includes an introductory tab and video for guidance. The simulation is designed to allow students to change parameters and explore.
- Exploration Parameters: Students can modify:
- Concentration of solutions.
- Type of reagent (monoprotic or diprotic acids/bases).
- Values of Ka (for weak acids) or Kb (for weak bases).
- Observational Learning: Students should note how parameter changes affect:
- The general shape of the curve.
- The volume required for complete neutralization.
- The pH at the point of complete neutralization.
4. Types of Titration Curves:
The resource emphasizes exploring various titration types:
- Strong acid – strong base
- Strong base – strong acid
- Weak acid – strong base
- Strong base – weak acid
- Weak base – strong acid
- Strong acid – weak base
5. Common Aspects of Titration Curves
The resource emphasizes that at the point of complete neutralization, specific trends can be observed, regardless of the curve type. These commonalities are not specifically elaborated on beyond asking the question, but the document notes that the volume required for neutralization stays the same when a strong acid is changed to a weak acid of the same concentration. The document also notes that the pH change at the point of complete neutralisation in a strong acid - weak base titration is approximately between 3 to 7.
6. Detailed Analysis of Specific Titration Curves:
- Strong Acid - Strong Base: The resource encourages careful study of the reaction mixture's composition at different points of the curve. Students are encouraged to perform pH calculations. The resource states, "Pay special attention to the species present at each point of the titration."
- Weak Acid - Strong Base: Similarly, the resource emphasizes the importance of species present throughout the titration and calculating pH at each stage. It also notes that the curve will change when a weak acid is used compared to a strong acid.
7. Key Points of Interest on a Weak Acid - Strong Base Titration Curve:
The resource provides detailed explanations for a titration of a strong base against a weak acid with specific reference to the example of ethanoic acid and sodium hydroxide. It breaks down the curve into five key points, labeled (i) to (v), and includes example calculations:
- (i) Initial pH: Calculated based on the concentration of the strong base. For example, with 0.100 mol dm-3 NaOH the initial pH = 13.
- (ii) pH Change during Neutralisation: The curve shows a sharp change in pH before and after the equivalence point.
- (iii) pH at Equivalence Point: The pH at the equivalence point is greater than 7 because a basic salt is formed. The resource explains the hydrolysis of the salt (CH3COO-). An example calculation shows the pH at the equivalence point in the ethanoic acid and sodium hydroxide example to be 8.70.
- (iv) Volume of Weak Acid Required to Reach Equivalence Point: This is determined using stoichiometry and noting that the weak acid and strong base will react in a 1:1 molar ratio. Using the ethanoic acid and sodium hydroxide example, with 10 cm3 of 0.100 mol dm-3 sodium hydroxide, 10 cm3 of 0.100 mol dm-3 ethanoic acid is required to reach the equivalence point.
- (v) Volume of Weak Acid Required for pH = pKa: This is the point of maximum buffering capacity and is reached when the concentrations of the weak acid and its conjugate base are equal. In the ethanoic acid and sodium hydroxide example, this point is reached after adding an additional 10 cm3 of ethanoic acid after the equivalence point.
8. Determining Ka:
The document contains a quiz question with associated feedback relating to a titration of a weak acid with a strong base. In this question, the value of Ka is determined from the knowledge that "When 10 cm3 of NaOH was added, half the HX has reacted to form salt. There is equal amount of excess HX and X- present and pH = pKa".
9. Skills and Career Relevance:
The document emphasizes that interpreting titration curves is a valuable skill for those considering higher-level science studies or careers in STEM fields. It offers training in analyzing graphs which is a key scientific skill.
10. Additional Resources:
The document contains a long list of other resources available on the Open Educational Resources / Open Source Physics @ Singapore platform, spanning diverse topics in physics and chemistry. It also details information about the licenses associated with the resources.
11. Conclusion:
The Titration Curve Generator appears to be a valuable interactive tool for learning about acid-base titrations. The resource is a great fit for students who are already familiar with some of the basic concepts and are ready to investigate more complex information. It allows for the exploration of different scenarios and provides detailed explanations of key points within the curves, enhancing conceptual understanding.
Titration Curves Study Guide
Quiz
Instructions: Answer the following questions in 2-3 sentences each.
- What is a titration curve, and what two variables are typically plotted on its axes?
- Describe the difference in shape between a titration curve of a strong acid with a strong base and one with a weak acid.
- What is the equivalence point of a titration, and how is it indicated on a titration curve?
- Explain how the pH at the equivalence point differs when a strong acid is titrated with a strong base compared to a weak acid titrated with a strong base.
- What is the significance of the point on a titration curve where pH = pKa?
- What does the term "titrant" refer to in the context of a titration?
- How can a titration curve be used to determine the concentration of an unknown solution?
- How does the concentration of the acid or base affect the titration curve?
- What chemical processes occur at the equivalence point of a weak acid and strong base titration?
- If you are titrating a weak acid, like ethanoic acid, using a strong base like NaOH, where will you find the buffer region on the titration curve?
Quiz Answer Key
- A titration curve is a graph that shows the change in pH of a solution as a titrant is added. The two variables plotted are pH on the y-axis and the volume of titrant added on the x-axis.
- The titration curve for a strong acid with a strong base shows a sharp, vertical change in pH near the equivalence point, while a weak acid curve has a more gradual slope and a less distinct inflection point.
- The equivalence point is the point in a titration where the acid and base have completely neutralized each other. It's indicated on the titration curve by the steepest slope or inflection point of the curve.
- At the equivalence point, the pH is 7 when a strong acid is titrated with a strong base. When a weak acid is titrated with a strong base, the pH at the equivalence point is greater than 7 due to the formation of a basic salt.
- When pH = pKa, the concentrations of the weak acid and its conjugate base are equal, creating a buffer solution at its maximum buffering capacity.
- The titrant is a solution with a known concentration that is added to a solution with an unknown concentration in order to determine the unknown solution's concentration.
- By analyzing the volume of titrant needed to reach the equivalence point and using stoichiometry, one can calculate the concentration of the unknown solution.
- Changing the concentrations will shift the y-axis values, for example, affecting the initial pH. But, for weak acids and strong bases, the volume of titrant required to reach the equivalence point will remain the same if the concentrations are proportionately scaled up or down.
- At the equivalence point of a weak acid and strong base titration, the salt of the weak acid is formed and undergoes hydrolysis, producing hydroxide ions (OH-) and making the solution basic.
- For the titration of a weak acid with a strong base, the buffer region will be formed after the equivalence point.
Essay Questions
Instructions: Answer the following questions in well-organized essays, using evidence from the source material.
- Discuss the importance of titration curves in chemistry, and how their interpretation can be used in a variety of scientific and industrial applications.
- Compare and contrast the titration curves produced when titrating strong acids and weak acids with strong bases, paying attention to the significant points on the curves and the underlying chemical principles.
- Explain in detail how the parameters of a titration—such as concentration, acid or base strength, and Ka or Kb values—affect the shape and characteristics of the resulting titration curve.
- Describe the chemical species present during different stages of a weak acid-strong base titration, and explain how the reaction mixture changes as titrant is added.
- Using the example of ethanoic acid titrated with sodium hydroxide provided in the material, walk through the calculations required to find the pH at various stages of the titration, including the equivalence point and the buffer region.
Glossary
Titration: A laboratory technique where a solution of known concentration (titrant) is added to a solution of unknown concentration until the reaction is complete, typically measured by a color change or a pH meter.
Titration Curve: A graph that plots the pH of a solution against the volume of titrant added during a titration.
Titrant: A solution of known concentration that is added during a titration to a solution of unknown concentration.
Equivalence Point: The point in a titration where the amount of titrant added is stoichiometrically equal to the amount of the substance being titrated. It is often indicated by a sudden change in pH or a color change.
Strong Acid: An acid that completely dissociates into ions when dissolved in water.
Strong Base: A base that completely dissociates into ions when dissolved in water.
Weak Acid: An acid that does not completely dissociate into ions in solution.
Weak Base: A base that does not completely dissociate into ions in solution.
Ka: The acid dissociation constant, which measures the strength of a weak acid. It is the equilibrium constant for the dissociation of an acid.
pKa: The negative logarithm of the acid dissociation constant (Ka). A lower pKa indicates a stronger acid.
Kb: The base dissociation constant, which measures the strength of a weak base. It is the equilibrium constant for the dissociation of a base.
pKb: The negative logarithm of the base dissociation constant (Kb). A lower pKb indicates a stronger base.
Kw: The ion product constant for water, equal to [H+][OH-]. At 25°C, Kw = 1.0 x 10^-14.
Buffer Solution: A solution that resists changes in pH when small amounts of acid or base are added. It typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
Hydrolysis: The chemical reaction in which a substance reacts with water.
Download model (simulator made by grace leong)
Titration Curves [H2 Chemistry]
About Introduction to Titration Curves https://library.opal.moe.edu.sg/ictc&func=view&rid=2348
Can you predict the shape of the titration curve obtained when a solution of NaOH was titrated against HCl?
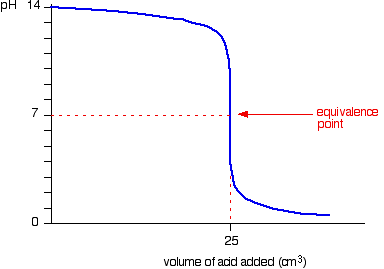
In this lesson, you will be working with a titration curve generator, a simulation to generate and explore the shapes of titration curves involving strong or weak acids and bases.
If you are not yet familiar with the usage of the titration curve generator, please read the introduction tab or watch this video to learn how to use it.
Exploring pH changes using the titration curve generator
Change the parameters and explore the features of the titration curve generator. Generate the shapes of the various titration curves
-
Strong acid - strong base
-
Strong base - strong acid
-
Weak acid - strong base
-
Strong base - weak acid
-
Weak base - strong acid
-
Strong acid - weak base
Change these parameters:
-
Concentration of solutions
-
Type of reagent - monoprotic or diprotic
-
Values of Ka (for weak acids) /Kb (for weak bases)
Observe what happens to the:
- General shape of curve
- Volume required for complete neutralisation
- pH at point of complete neutralisation
What is common across all titration curves at the point of complete neutralisation?
When a strong acid is changed to a weak acid of the same concentration, the volume of the same base required for complete neutralisation
stays the same
The approximate pH change at the point of complete neutralisation for a strong acid - weak base titration is from
3 to 7
Strong acid - Strong base titration curve
Weak acid - Strong base titration curve
Check your understanding Sketch on a piece of paper, the titration curve obtained when 20 cm3 of 0.100 mol dm-3 ethanoic acid (pKa = 4.7) is added to 10.0 cm3 of 0.100 mol dm-3 sodium hydroxide. In your sketch, pay attention to the initial pH, pH at equivalence point ,volume of ethanoic acid required to reach equivalence point, volume of ethanoic acid required for pH = pKa.
pH = 14 - 1.00 = 13
Amount of ethanoic acid = 1.00 x 10-3 mol
Volume of ethanoic acid = http://www.w3.org/1998/Math/MathML">1×10-30.100" class="Wirisformula" role="math" alt="fraction numerator 1 cross times 10 to the power of negative 3 end exponent over denominator 0.100 end fraction" style="box-sizing: border-box; min-width: auto; min-height: auto; padding: 0px; margin: 0px; outline: none; display: inline-block; vertical-align: -12px; border: none; height: 40px; width: 73px;"> = 10 cm3
Concentration of sodium ethanoate = http://www.w3.org/1998/Math/MathML">1×10-3201000" class="Wirisformula" role="math" alt="fraction numerator 1 cross times 10 to the power of negative 3 end exponent over denominator begin display style bevelled 20 over 1000 end style end fraction" style="box-sizing: border-box; min-width: auto; min-height: auto; padding: 0px; margin: 0px; outline: none; display: inline-block; vertical-align: -19px; border: none; height: 47px; width: 76px;"> = 0.05 mol dm-3
What is the value of Ka, in mol dm−3, for this acid HX?
1.0 × 10−5
Translation
[text]
Research
[text]
Video
[text]
Credits
[SIMU_CREDITS]
Version:
[text]
Other Resources
[text]
Titration Curves: Frequently Asked Questions
- What is a titration curve, and what information does it provide?
- A titration curve is a graph that plots the pH of a solution against the volume of a titrant (a solution of known concentration) added during a titration. Titration is a technique where a titrant is slowly added to a solution of unknown concentration in order to find its concentration. The curve visually represents how the pH of the solution changes as the titrant is added, revealing important information about the reaction between the titrant and the substance in the solution, including the equivalence point (neutralization point) of the titration.
- What are the key differences between titration curves for strong acids/bases versus weak acids/bases?
- Titration curves for strong acids and bases typically exhibit a very sharp and large change in pH near the equivalence point, reflecting the complete dissociation of both reactants. The equivalence point for strong acid-strong base titrations occurs at pH 7. Titration curves involving weak acids or bases demonstrate a more gradual pH change near the equivalence point and the pH at the equivalence point is not always at 7. Additionally, weak acid/base titrations show the presence of a "buffer region" where pH changes are relatively small. Weak acid/strong base titrations have an equivalence point at a pH greater than 7, and weak base/strong acid titrations have an equivalence point less than 7.
- What is the "equivalence point" on a titration curve, and how is it determined?
- The equivalence point, also known as the neutralization point, is the point in a titration where the titrant has completely reacted with the substance in the solution. On a titration curve, this point is characterized by a vertical or near vertical inflection point. The steepness of the pH change near the equivalence point helps in identifying its exact location. At the equivalence point, the moles of acid equals the moles of base in the flask.
- How can you use a titration curve to determine the strength of an acid or base?
- By analyzing the shape and characteristics of the titration curve, such as the sharpness of the pH change at the equivalence point and the pH at different regions of the curve, we can infer the strengths of acids and bases. A sharp change in pH suggests that both the titrant and the substance are either strong acids or bases. A more gradual change indicates that the substance being titrated is either a weak acid or a weak base, where the dissociation is incomplete. The presence of a buffer region also indicates the presence of a weak acid or base.
- What is a buffer region, and how does it appear on a titration curve?
- A buffer region is the part of the titration curve where the pH of the solution changes only slightly when more titrant is added. It is the region where both a weak acid and its conjugate base (or a weak base and its conjugate acid) are present in significant amounts. This region occurs when titrating a weak acid or a weak base. The buffer region appears on a titration curve as a relatively flat section before the equivalence point. The greatest buffering capacity is achieved when the pH is equal to the pKa of the weak acid.
- How do you calculate the pH of a solution at the equivalence point for a weak acid-strong base titration?
- At the equivalence point of a weak acid-strong base titration, the solution will have only the conjugate base and water present. This salt formed will hydrolyze in water creating hydroxide ions, which causes the pH to be greater than 7. The pH can be calculated by considering the hydrolysis of the conjugate base and using the Kb for this reaction and the concentration of the conjugate base at the equivalence point to calculate the concentration of the hydroxide ion and subsequently the pH of the solution. The steps are to first calculate the moles of base at the start of the reaction which is equal to the moles of acid reacted at the equivalence point. Then determine the volume of the solution at the equivalence point, and finally calculate the concentration of the conjugate base. The rest can be found using the equilibrium expression of the hydrolysis of the conjugate base.
- How does the Ka value of a weak acid relate to the pH of the solution during a titration?
- The acid dissociation constant (Ka) indicates the strength of a weak acid. A lower Ka indicates a weaker acid and therefore more gradual pH change at the start of the titration of a weak acid. When the pH of the solution equals the pKa of the weak acid (where pKa = -log Ka), the concentration of the weak acid is equal to the concentration of the conjugate base. This point is located in the buffer region of the titration curve where it has the highest buffering capacity and is located at half the volume at the equivalence point of a weak acid/strong base titration.
- Why is the ability to read and interpret titration curves considered an important skill in science and STEM careers?
- Titration curves, like other graphs used in science, are visual representations of data and trends and understanding them is crucial for the development of analytical skills. The ability to read, analyze, and interpret titration curves is a necessary skill in many science fields where titrations are commonly used, such as in medical or food industries, or in chemical research to understand acid-base reactions, determine concentrations of unknown substances, and for overall critical thinking in STEM careers.

