About
Adding Heat
Introduction
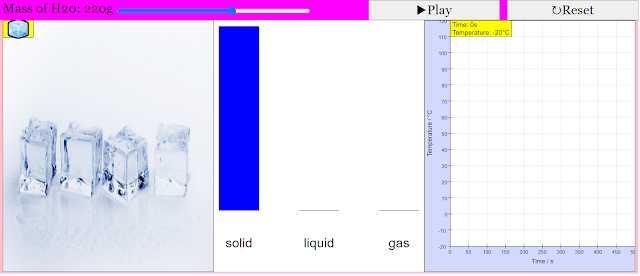
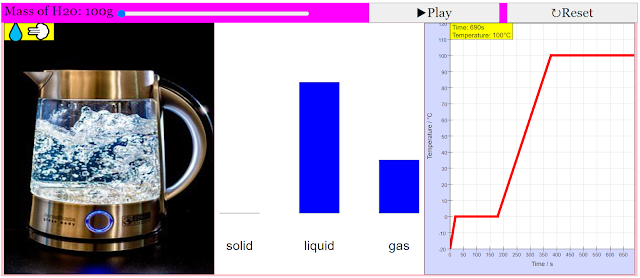
The purpose of this simulation is to show how the temperature of water changes over time, as it undergoes phase transitions, when supplied with energy at a constant rate.Description of simulation
This simluation features both a graph of temperature over time, as well as a 'world view' to facillitate the students' understanding of how the shape of the water changes as it undergoes various phase transitions.Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits
Shaun Quek; lookang; inspired by a version from Andrew Duffy; http://physics.bu.edu/~duffy/HTML5/heat_addHeat.html
Overview
This briefing document reviews a JavaScript HTML5 applet simulation model designed to illustrate the changes in temperature of water over time as it absorbs heat at a constant rate and undergoes phase transitions (solid to liquid, liquid to gas). The simulation provides a visual and interactive tool for understanding the thermal properties of matter, heat transfer, and the concept of temperature.
Main Themes and Important Ideas
1. Visualizing Phase Transitions with Constant Heat Input:
- The primary purpose of the simulation is to demonstrate how the temperature of water changes as energy is added at a constant rate, specifically focusing on the plateaus in temperature that occur during melting (solid to liquid) and boiling (liquid to gas).
- The simulation uses a graph of temperature over time to clearly show these temperature plateaus, indicating that during a phase change, the added energy goes into breaking intermolecular bonds rather than increasing the kinetic energy of the molecules (and thus the temperature).
- A "world view" panel is included to provide a visual representation of the water sample changing its physical state (ice, liquid water, steam) alongside the temperature graph. This helps students connect the macroscopic changes they observe with the microscopic processes occurring.
Quote: "The purpose of this simulation is to show how the temperature of water changes over time, as it undergoes phase transitions, when supplied with energy at a constant rate."
2. Quantitative Relationship between Heat Input and Phase Change:
- The simulation allows users to observe how the mass of water in each phase (solid, liquid, gas) changes over time using a bar chart. This visual representation reinforces the idea that as heat is added during a phase transition, the proportion of the substance in each phase shifts.
- The accompanying text highlights the potential for quantitative analysis: "With these numbers and the information from the graph, you should be able to figure out the rate at which energy is added to the water sample." This suggests that students can use the data presented in the simulation (temperature, time, mass changes) to calculate the rate of heat transfer and potentially latent heats of fusion and vaporization.
- The simulation starts with water at -20°C and allows the total mass of water to be adjusted (default 100g, maximum 300g via a slider). This variability enables exploration of how the mass of the substance affects the duration of the temperature increase and the phase transitions.
Quote: "When the simulation is started, the temperature of water increases with time. However, as the water transitions between states (from sold to liquid, and liquid to gas), the temperature of water remains constant with time. The bar chart would also illustrate how the mass of water changes between states."
3. Emphasizing the Time Spent During Phase Change:
- The description explicitly draws attention to the significant amount of energy (and therefore time, with a constant heating rate) required to complete a phase transition compared to the time it takes to raise the temperature within a single phase.
- The text encourages users to "Don't forget to be amazed by how much time the sample spends changing phase." This pedagogical approach aims to highlight a key concept often misunderstood by students – that a substantial amount of energy is involved in changing the state of matter.
Quote: "Don't forget to be amazed by how much time the sample spends changing phase."
4. Pedagogical Tool for Teaching Thermal Physics:
- The resource is explicitly presented as an "Open Educational Resource" intended for teaching thermal physics concepts, specifically "Transfer of thermal energy," "Temperature," and "Thermal Properties of Matter."
- The inclusion of "Sample Learning Goals" (though the specific goals are not provided in the excerpt) and a section "For Teachers" indicates its intended use in educational settings.
- The simulation is designed to "facillitate the students' understanding" of phase transitions through its combined graphical and visual representations.
Quote: "This simluation features both a graph of temperature over time, as well as a 'world view' to facillitate the students' understanding of how the shape of the water changes as it undergoes various phase transitions."
5. Technical Details and Credits:
- The simulation is a "2021 JavaScript HTML5 Applet Simulation Model," indicating its accessibility through web browsers without the need for additional plugins.
- Credits are given to Shaun Quek and lookang, with inspiration from an earlier version by Andrew Duffy. This acknowledges the contributions of the developers and provides a link to the original inspiration.
- The model was "Compiled with EJS 6.02_BETA (201222)," referencing the Easy Java/JavaScript Simulations Toolkit used in its creation.
Conclusion
The "Adding Heat to Water" simulation is a valuable educational tool for visualizing and understanding the relationship between heat input, temperature change, and phase transitions in water. By providing interactive graphical and visual representations, it aims to enhance student comprehension of key thermal physics concepts and encourages quantitative analysis of the energy involved in these processes. The emphasis on the time spent during phase changes highlights a crucial aspect of the thermal behavior of matter. As an open educational resource, it offers accessibility for educators to integrate into their teaching materials.
Study Guide: Adding Heat to Water Simulation
I. Key Concepts
- Thermal Energy Transfer: The process by which heat moves from a warmer object or system to a cooler one. In this simulation, energy is added to the water at a constant rate.
- Temperature: A measure of the average kinetic energy of the particles within a substance. Temperature changes when thermal energy is added or removed, except during a phase transition.
- Phase Transition: The physical process of a substance changing from one state of matter to another (solid, liquid, gas). The simulation focuses on melting (solid to liquid) and boiling (liquid to gas) of water.
- States of Matter: The distinct forms that matter can take. In this simulation, the three states are solid (ice), liquid (water), and gas (water vapor/steam).
- Constant Rate of Energy Input: Energy is supplied to the water at a steady, unchanging rate throughout the simulation. This allows for the calculation of the amount of energy required for temperature changes and phase transitions based on the time taken.
- Melting Point: The specific temperature at which a solid substance changes to a liquid (for water, 0°C at standard pressure). During melting, added energy goes into breaking the bonds holding the solid structure, not increasing temperature.
- Boiling Point: The specific temperature at which a liquid substance changes to a gas (for water, 100°C at standard pressure). During boiling, added energy goes into overcoming intermolecular forces to allow molecules to escape into the gaseous phase, not increasing temperature.
- Heat Capacity: The amount of heat energy required to raise the temperature of a substance by a specific amount (e.g., one degree Celsius per gram). Different phases of water (solid, liquid, gas) have different heat capacities.
- Latent Heat: The energy absorbed or released by a substance during a phase change at a constant temperature. There is latent heat of fusion (during melting or freezing) and latent heat of vaporization (during boiling or condensation).
II. Using the Simulation
- Interface: The simulation typically features:
- A graph showing temperature versus time.
- A "world view" illustrating the physical state of the water (ice, liquid water, steam).
- A bar chart showing the mass of water in each state (solid, liquid, gas).
- A slider to adjust the initial mass of the water sample (up to 300g).
- Observation: Observe how the temperature changes over time as energy is added. Notice the periods when the temperature increases and the periods when it remains constant despite the ongoing addition of heat.
- Phase Changes: Pay attention to how the "world view" and the bar chart change during the constant temperature periods. These correspond to the melting of ice and the boiling of liquid water.
- Data Analysis: The graph provides data on the time taken for each phase (temperature increase, melting, temperature increase, boiling). This data, along with the known mass of water and the constant rate of energy input, can be used to calculate thermal properties of water.
- Learning Goals: The simulation aims to help understand:
- How temperature changes with constant energy input.
- The concept of phase transitions and their effect on temperature.
- The difference in energy behavior during temperature changes and phase transitions.
- The relative amount of time spent in each phase change compared to temperature changes.
III. Quiz
- What is the primary purpose of the "Adding Heat to Water" simulation?
- Describe what happens to the temperature of the water during a phase transition (like melting or boiling) when heat is continuously added.
- What are the three panels typically displayed in the simulation, and what does each illustrate?
- According to the simulation description, what is the initial state of the water sample by default? What adjustable parameter is mentioned?
- Explain why the temperature remains constant during a phase change even though energy is still being added to the water.
- What can you determine about the rate of energy addition to the water sample by observing the simulation's graph and knowing the mass of the water?
- What are the two main phase transitions involving water that are demonstrated in this simulation?
- How does the bar chart in the simulation help visualize the phase transitions of the water?
- Besides the simulation itself, what other types of learning resources or information are associated with this model on the webpage?
- Who are credited as the creators or inspirers of this "Adding Heat to Water" simulation?
IV. Quiz Answer Key
- The primary purpose of the simulation is to show how the temperature of water changes over time, as it undergoes phase transitions (melting and boiling), when supplied with energy at a constant rate. It visually demonstrates the relationship between heat addition, temperature change, and changes in the state of matter.
- During a phase transition, the temperature of the water remains constant even though heat is continuously added. This added energy is used to break the intermolecular bonds or change the potential energy of the molecules, rather than increasing their kinetic energy (which would raise the temperature).
- The three panels typically displayed are: a "real world" view for illustration, a bar chart showing the mass of water in solid, liquid, and gas states, and a graph of temperature as a function of time. Each panel provides a different representation of the process.
- By default, the initial state of the water sample is at -20°C. The slider at the top right allows the user to alter the total mass of water, with a maximum of 300g.
- The temperature remains constant during a phase change because the energy being added is used to overcome the forces holding the molecules in their current state (solid or liquid). This energy input facilitates the transition to a higher energy state (liquid or gas) without increasing the average kinetic energy of the molecules.
- By analyzing the slope of the temperature-versus-time graph during periods where the water is in a single phase (solid, liquid, or gas) and knowing the mass of the water and its specific heat capacity, one can calculate the rate at which energy is being added. The duration of the phase changes can also help determine the energy required for these transitions.
- The two main phase transitions involving water demonstrated in this simulation are melting, which is the transition from solid (ice) to liquid (water), and boiling, which is the transition from liquid (water) to gas (water vapor/steam).
- The bar chart dynamically illustrates the phase transitions by showing the changing mass of water in each state (solid, liquid, and gas) over time. As ice melts, the solid bar decreases and the liquid bar increases, and similarly for the transition from liquid to gas.
- Besides the simulation, the webpage includes information about the model's description, credits, sample learning goals, resources for teachers, translations, links to research and video materials, and versions on other platforms. It also lists other related interactive simulations and tools.
- Shaun Quek and lookang are credited with the 2021 JavaScript HTML5 Applet Simulation Model, inspired by a version from Andrew Duffy (http://physics.bu.edu/~duffy/HTML5/heat_addHeat.html).
V. Essay Format Questions
- Discuss the relationship between the addition of heat energy to a substance and its temperature, specifically addressing why the temperature does not continuously increase when a substance undergoes a phase transition. Use the "Adding Heat to Water" simulation as a concrete example to support your explanation.
- Explain the significance of latent heat in the context of phase transitions. How does the "Adding Heat to Water" simulation visually represent the concept of latent heat, and why is it important in understanding the energy requirements for changes of state?
- Compare and contrast the behavior of water's temperature change when it is in a single phase (solid, liquid, or gas) versus when it is undergoing a phase transition. Analyze how the "Adding Heat to Water" simulation effectively demonstrates these differences.
- Describe the various components of the "Adding Heat to Water" simulation interface and explain how each component contributes to a student's understanding of the process of adding heat to water and its resulting temperature changes and phase transitions.
- Considering the information provided about the "Adding Heat to Water" simulation, discuss its potential value as an educational tool for teaching concepts related to thermal energy, temperature, and the properties of matter. What are some specific learning objectives that this simulation could help students achieve?
VI. Glossary of Key Terms
- Thermal Energy: The total internal energy of a system due to the kinetic and potential energy of its atoms and molecules. Often referred to as heat.
- Temperature: A measure of the average kinetic energy of the particles in a substance. It determines the direction of heat flow between two objects in thermal contact.
- Phase: A physically distinct form of matter, such as solid, liquid, or gas, having uniform chemical composition and physical properties.
- Melting: The phase transition of a substance from a solid to a liquid state.
- Boiling: The phase transition of a substance from a liquid to a gaseous state, typically occurring when the vapor pressure of the liquid equals the surrounding pressure.
- Freezing: The phase transition of a substance from a liquid to a solid state. The reverse of melting.
- Condensation: The phase transition of a substance from a gaseous to a liquid state. The reverse of boiling.
- Sublimation: The phase transition of a substance directly from a solid to a gaseous state, without passing through the liquid phase.
- Deposition: The phase transition of a substance directly from a gaseous to a solid state, without passing through the liquid phase.
- Specific Heat Capacity: The amount of heat required to raise the temperature of one unit mass of a substance by one degree Celsius (or Kelvin).
- Latent Heat of Fusion: The amount of heat energy absorbed or released per unit mass during the process of melting or freezing at a constant temperature and pressure.
- Latent Heat of Vaporization: The amount of heat energy absorbed or released per unit mass during the process of boiling or condensation at a constant temperature and pressure.
- Constant Rate: Occurring or proceeding without change in speed or amount over time. In this context, energy is added to the water at a steady pace.
Sample Learning Goals
[text]
For Teachers
Adding Heat to Water 2021 JavaScript HTML5 Applet Simulation Model
Description of the model
Research
[text]
Video
[text]
Version:
Other Resources
http://physics.bu.edu/~duffy/HTML5/heat_addHeat.html
Frequently Asked Questions: Heating Water and Phase Transitions
- What is the purpose of the "Adding Heat to Water" simulation? The primary goal of this simulation is to visually demonstrate how the temperature of a water sample changes over time as it absorbs energy at a constant rate and undergoes transitions between its different phases: solid (ice), liquid (water), and gas (steam).
- How does the simulation illustrate the process of adding heat to water? The simulation provides a graph showing the temperature of the water over time. Additionally, it includes a "world view" that visually represents the physical state of the water, and a bar chart indicating the mass of water in each phase (solid, liquid, and gas) as energy is continuously added.
- What happens to the temperature of the water during a phase transition (melting or boiling)? A key observation from the simulation is that during a phase transition (when ice is melting into water, or when water is boiling into steam), the temperature of the water remains constant even though energy is still being added. The added energy at these points is used to break the intermolecular bonds and change the state, rather than increase the kinetic energy of the molecules (which would result in a temperature increase).
- What initial conditions are set in the simulation? By default, the simulation starts with a 100-gram sample of water at an initial temperature of -20°C (solid ice). However, users can adjust the initial mass of the water using a slider, up to a maximum of 300 grams.
- What information can be derived from observing the simulation? By observing the temperature vs. time graph and the changes in the bar chart representing the different phases, users can understand the relationship between energy input, temperature change, and phase transitions. They can also determine the rate at which energy is being added to the water sample by analyzing the slope of the temperature increase in each phase and the duration of the phase transitions.
- What are the different visual components of the simulation? The simulation features three main panels: a "real world" view for illustrative purposes showing the water in its current phase, a bar chart that dynamically updates to show the mass of water in the solid, liquid, and gas states, and a graph displaying the temperature of the water as a function of time.
- What are some potential learning goals or takeaways from using this simulation? Students can gain a better understanding of concepts such as heat transfer, temperature, phase transitions (melting, freezing, boiling, condensation), latent heat (the energy absorbed or released during a phase change at a constant temperature), and the relationship between energy input and changes in the state of matter.
- Who developed this simulation and where can I find more information or similar resources? This "Adding Heat to Water" simulation was developed by Shaun Quek and lookang, inspired by a version from Andrew Duffy (http://physics.bu.edu/~duffy/HTML5/heat_addHeat.html). It is part of the Open Educational Resources / Open Source Physics @ Singapore project. The source code and related resources can be found on their website and potentially through the provided links. The Easy Java/JavaScript Simulations (EJS) Toolkit by Francisco Esquembre and Félix Jesús Garcia Clemente is also mentioned as a tool used in the creation of such simulations.
- Details
- Written by Shaun
- Parent Category: 13 Thermodynamic Systems
- Category: 04 Thermal Properties of Matter
- Hits: 8682


.png
)