About
Mass spectrometry
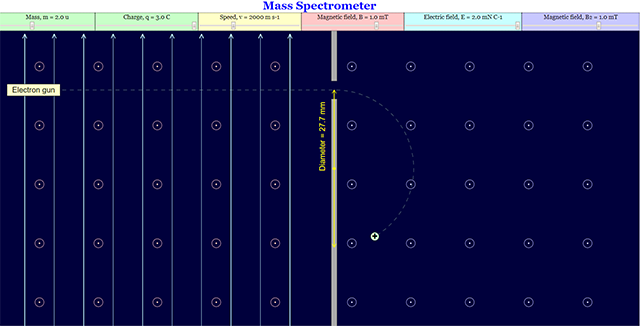
This is the simulation of a mass spectrometer where students are allowed to manipulates mass, charge, speed, magnetic field, electric field
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits
 Leong Tze Kwang
Leong Tze Kwang
Main Themes:
- The Mass Spectrometer as a Tool for Scientific Inquiry: Both sources highlight the mass spectrometer as an instrument used in science. The applet explicitly states its function as a "simulation of a mass spectrometer where students are allowed to manipulates mass, charge, speed, magnetic field, electric field." This emphasizes the device's role in allowing the investigation of the behavior of charged particles under the influence of electromagnetic fields.
- Historical Development of the Atomic Model: The "Mass Spectrometer HTML5 Applet Javascript" resource provides a significant historical context, tracing the evolution of our understanding of the atom from Dalton's indivisible spheres to Thomson's discovery of the electron and Rutherford's discovery of the nucleus. This historical backdrop underscores the importance of tools like the mass spectrometer in furthering our knowledge of matter at the atomic level.
- Fundamental Principles of Physics: The ability of the mass spectrometer to separate particles based on their mass-to-charge ratio relies on fundamental principles of electromagnetism, specifically the forces experienced by charged particles in electric and magnetic fields (Lorentz force). The resource mentions the deflection of cathode rays (electrons) by electric and magnetic fields, a key discovery that paved the way for understanding charged particles and ultimately the principles behind mass spectrometry.
- Educational Applications of Simulations: The inclusion of an HTML5 applet demonstrates the use of technology and simulations as educational tools in physics. By allowing students to manipulate variables, the simulation provides an interactive way to understand the workings of a mass spectrometer and the factors influencing the paths of charged particles.
- The Nature of Scientific Inquiry: The introductory section of the "Mass Spectrometer HTML5 Applet Javascript" resource touches upon the core of scientific inquiry, stating: "In science, unlike in polite conversation, we are quite accustomed to questioning assumptions and the question 'Why?' can usually be translated as 'How do you know?'" This sets the stage for understanding how scientific models, like that of the atom, evolve through questioning, experimentation, and evidence.
Most Important Ideas and Facts:
- Mass Spectrometry Basics: The core idea is that a mass spectrometer separates ions based on their mass-to-charge ratio. While the provided excerpts don't detail the exact mechanisms within the "Mass Spectrometer" document itself, the applet's description implies the use of electric and magnetic fields to achieve this separation.
- Dalton's Atomic Theory (Early 1800s): Dalton proposed that "Gases are made up of small particles, which are referred to as atoms," that "All atoms of a given element are identical," and that "The atoms of different elements vary by mass and other properties." His work provided a foundational model for understanding chemical processes.
- Thomson's Discovery of the Electron (1897): J. J. Thomson's experiments with cathode rays revealed that atoms were not indivisible. He discovered negatively charged particles (electrons) that could be deflected by electric and magnetic fields. This led to his "plum-pudding model" where electrons were embedded in a positively charged sphere.
- Rutherford's Discovery of the Atomic Nucleus (1909): Rutherford's gold foil experiment demonstrated that most of an atom's mass and positive charge were concentrated in a small, dense nucleus. He observed that "most of the alpha particles pass through with small deflections... But some of these particles bounce right back!" This led to the planetary model of the atom with electrons orbiting a central nucleus.
- The Role of Electric and Magnetic Fields: Thomson's work showed that charged particles are deflected by both electric and magnetic fields, a principle crucial to the operation of a mass spectrometer. The applet allows users to "enable the view of the Electric Field on the left side" and "change the view of the Magnetic Field on both sides," highlighting the importance of these fields in manipulating the trajectory of ions.
- Simulations as Learning Tools: The "Mass Spectrometer HTML5 Applet Javascript" is designed to allow students to "manipulates mass, charge, speed, magnetic field, electric field" to understand the relationship between these variables and the behavior of ions in a mass spectrometer. This interactive approach is intended to enhance learning in physics.
Quotes from the Original Sources:
- About the Mass Spectrometer Simulation: "This is the simulation of a mass spectrometer where students are allowed to manipulates mass, charge, speed, magnetic field, electric field" (Open Educational Resources / Open Source Physics @ Singapore).
- On Questioning Assumptions in Science: "'Why?' can usually be translated as 'How do you know?'" (Open Educational Resources / Open Source Physics @ Singapore).
- Dalton's Atomic Model: "Gases are made up of small particles, which are referred to as atoms. All atoms of a given element are identical. The atoms of different elements vary by mass and other properties." (Open Educational Resources / Open Source Physics @ Singapore, describing Dalton's proposal).
- Thomson's Discovery: "When experimenting with the phenomenon of 'cathode rays', J. J. Thomson discovered that these rays were made of charged particles (we call them electrons) because they could be deflected by electric and magnetic fields." (Open Educational Resources / Open Source Physics @ Singapore).
- Rutherford's Observation: "But some of these particles bounce right back!" (Open Educational Resources / Open Source Physics @ Singapore, describing the alpha particle scattering experiment).
- Rutherford's Deduction: "Rutherford deduced that the atoms must have their mass concentrated in a tiny volume (the 'nucleus'), such that most alpha particles do not pass very near these concentrations, but some alpha particles happen to pass very near and this basically results in an elastic collision between a relatively low-mass alpha particle and a relatively high-mass gold nucleus!" (Open Educational Resources / Open Source Physics @ Singapore).
Conclusion:
The provided sources, while one being a simple title and author attribution, collectively offer a valuable insight into the mass spectrometer as a scientific tool and its place within the historical development of atomic physics. The inclusion of an interactive simulation underscores the modern approach to science education, leveraging technology to provide hands-on learning experiences. The historical narrative reminds us that our current understanding of matter is a result of continuous questioning, experimentation, and the development of increasingly sophisticated tools like the mass spectrometer.
Mass Spectrometer Study Guide
Overview: This study guide reviews the principles behind the mass spectrometer and its historical context, including the development of the atomic model.
Key Concepts:
- Atomic Model Development: Understand the progression of atomic models from Dalton to Thomson to Rutherford, including the experiments and evidence that supported each model.
- Electrons: Know the properties of electrons as discovered by Thomson (charge, deflection by electric and magnetic fields, mass-to-charge ratio).
- Atomic Nucleus: Understand Rutherford's alpha-particle scattering experiment and the conclusions drawn about the existence, small size, and dense nature of the atomic nucleus.
- Ions: Understand how atoms become charged ions through the gain or loss of electrons.
- Mass Spectrometer: Comprehend the basic principle of operation of a mass spectrometer: using electric and magnetic fields to manipulate and separate charged particles based on their mass-to-charge ratio.
- Variables Affecting Ion Path: Identify the key variables that influence the path of ions within a mass spectrometer (mass, charge, speed, magnetic field strength, electric field strength).
- Applications of Mass Spectrometry: (Though not explicitly detailed in the provided text, consider broader knowledge of what mass spectrometers are used for, such as identifying isotopes and determining molecular weights).
Review Questions:
- Dalton's Atomic Theory: What were the main postulates of John Dalton's atomic theory as described in the text? What experimental evidence did he use to support his ideas?
- Thomson's Cathode Ray Experiment: Describe J.J. Thomson's experiment with cathode rays. What conclusions did he draw from his observations about the nature of these rays and the structure of the atom?
- Plum-Pudding Model: Explain Thomson's plum-pudding model of the atom. What were its key features, and how did it account for the neutrality of atoms?
- Rutherford's Alpha-Particle Scattering Experiment: Describe the setup and key observations of Rutherford's gold foil experiment. What was unexpected about the results?
- Discovery of the Nucleus: How did Rutherford interpret the results of his experiment? What conclusions did he make about the structure of the atom based on these observations?
- Planetary Model of the Atom: Explain Rutherford's planetary model of the atom. How did it differ from the plum-pudding model?
- Role of Electric and Magnetic Fields: According to the applet description, how can users manipulate electric and magnetic fields in the mass spectrometer simulation? What effect do these fields have on charged particles?
- Variables in the Mass Spectrometer: List the variables that users can change in the mass spectrometer applet simulation. How do these variables relate to the movement of ions within the instrument?
- Ions and Charge: How are ions formed from neutral atoms according to the text? What is the relationship between the loss or gain of electrons and the charge of the resulting ion?
- Significance of Discoveries: How did the discoveries of Thomson and Rutherford fundamentally change our understanding of the atom compared to Dalton's initial model?
Quiz:
- Describe one key piece of experimental evidence that supported Dalton's idea that atoms are fundamental, unchanging units in chemical reactions. Explain how this evidence led to his conclusion.
- What was the significance of Thomson's discovery that cathode rays could be deflected by electric and magnetic fields? How did this finding challenge the prevailing view of the atom?
- Explain the key difference between Thomson's plum-pudding model and Rutherford's planetary model of the atom. What observation from Rutherford's experiment led to this revised model?
- In Rutherford's gold foil experiment, most alpha particles passed through the gold foil with little to no deflection. What did this observation suggest about the structure of the atom?
- What caused some of the alpha particles in Rutherford's experiment to bounce back from the gold foil? What property of the atom did this reveal?
- According to the description of the mass spectrometer applet, what are two ways in which a user can alter the behavior of ions within the simulated instrument?
- Explain how the charge of an ion affects its behavior when it passes through a magnetic field in a mass spectrometer. Use the concept of the Lorentz force in your explanation.
- What is the role of electric fields in some designs of mass spectrometers, particularly in relation to controlling the speed of the ions?
- Why is it important to consider the mass-to-charge ratio (rather than just mass or charge alone) when analyzing the behavior of ions in a mass spectrometer?
- Briefly explain how the historical progression from Dalton's model to Rutherford's model demonstrates the self-correcting nature of the scientific process.
Quiz Answer Key:
- Dalton's observation of the law of partial pressures, where the total pressure of a gas mixture is the sum of individual gas pressures, suggested that gases are made of discrete particles (atoms) that retain their identity in mixtures. His work on the relative masses of gases in compounds further supported the idea of fixed ratios of these unchanging atomic units.
- The deflection of cathode rays by electric and magnetic fields demonstrated that these rays were composed of charged particles (electrons), which were constituents of atoms. This challenged the idea that atoms were indivisible and the smallest units of matter.
- The plum-pudding model proposed a diffuse positive charge with negatively charged electrons embedded within it, like plums in a pudding. Rutherford's experiment, where some alpha particles bounced back from a gold foil, led to the planetary model with a small, dense, positively charged nucleus at the center and electrons orbiting it.
- The fact that most alpha particles passed through the gold foil relatively unimpeded indicated that atoms were mostly empty space. This suggested that the atom's mass and positive charge were not uniformly distributed.
- The bouncing back of some alpha particles was due to their direct collision with a very small, dense, and positively charged region within the gold atoms, which Rutherford named the nucleus. This revealed that most of the atom's mass and all of its positive charge were concentrated in this tiny volume.
- Users can change the strength or direction of the magnetic field and enable/disable or change the strength/direction of the electric field. These changes will affect the force experienced by the charged particles, altering their trajectory.
- A moving charged particle experiences a force in a magnetic field (Lorentz force) that is perpendicular to both its velocity and the magnetic field direction. The magnitude of this force is proportional to the charge of the ion; a higher charge will result in a greater deflecting force for the same velocity and magnetic field strength, leading to a tighter curve in its path.
- Electric fields can be used to accelerate ions to a specific kinetic energy and thus a specific speed before they enter the magnetic field region of the mass spectrometer. This ensures that ions with the same mass-to-charge ratio will follow the same path in the magnetic field, regardless of slight variations in their initial speeds.
- The mass spectrometer separates ions based on the force they experience in magnetic and electric fields, which depends on both their charge and their motion. The motion, in turn, is influenced by their mass (inertia). Therefore, the resulting trajectory and separation are determined by the ratio of their mass to their charge (m/q).
- The progression from Dalton's indivisible atom to Thomson's atom with electrons, and finally to Rutherford's atom with a nucleus, illustrates how new experimental evidence can challenge existing theories. Rutherford's unexpected results forced a revision of Thomson's model, leading to a more accurate understanding of atomic structure.
Essay Format Questions:
- Trace the historical development of the atomic model from Dalton to Rutherford. Discuss the key experiments and the scientists involved in each stage, highlighting how each new model built upon or challenged the previous one.
- Explain the significance of J.J. Thomson's cathode ray experiment in revolutionizing our understanding of matter. Detail the experimental setup, observations, and the key conclusions drawn about the nature of atoms.
- Describe Rutherford's alpha-particle scattering experiment and analyze the unexpected results that led to the discovery of the atomic nucleus. Discuss how this discovery fundamentally changed the prevailing model of the atom.
- Based on the information provided about the mass spectrometer applet and the historical context of atomic discoveries, discuss the fundamental principles behind how a mass spectrometer separates ions. Consider the roles of electric and magnetic fields and the properties of the ions themselves.
- The development of the atomic model is a prime example of the scientific method in action. Discuss how the work of Dalton, Thomson, and Rutherford exemplifies the iterative process of observation, hypothesis formation, experimentation, and revision in scientific understanding.
Glossary of Key Terms:
- Atom: The basic unit of a chemical element, consisting of a central nucleus surrounded by electrons. Historically considered indivisible until the discovery of subatomic particles.
- Electron: A stable subatomic particle with a negative electric charge, found in all atoms and acting independently or as part of an atom.
- Cathode Rays: Streams of electrons observed in vacuum tubes, so named because they are emitted from the negative electrode, or cathode.
- Ion: An atom or molecule in which the total number of electrons is not equal to the total number of protons, giving the atom or molecule a net positive or negative electrical charge.
- Nucleus (atomic): The small, dense region at the center of an atom, consisting of positively charged protons and electrically neutral neutrons (except for the hydrogen-1 nucleus, which is just a proton).
- Alpha Particle: A helium nucleus consisting of two protons and two neutrons, emitted in some forms of radioactive decay. Used by Rutherford in his scattering experiment.
- Mass-to-Charge Ratio (m/q): A physical quantity relating the mass and the electric charge of a particle. It is frequently used in mass spectrometry and the physics of charged particles in electric and magnetic fields.
- Lorentz Force: The force exerted on a charged particle in an electromagnetic field. It is the vector sum of the electric and magnetic forces.
- Plum-Pudding Model: An obsolete scientific model of the atom proposed by J.J. Thomson before the discovery of the atomic nucleus. In this model, the atom is composed of electrons surrounded by a soup of positive charge to balance the electrons' negative charges, like plums in a pudding.
- Planetary Model: An early model of the atom proposed by Ernest Rutherford, in which electrons orbit a central, positively charged nucleus, much like planets orbiting a star.
Sample Learning Goals
Topic/Sub-Topics
-
20(a) infer from the results of the Rutherford α-particle scattering experiment the existence and small size of the atomic nucleus
For Teachers
SLS Lesson History of the atomic model by Leong T.K.
-
Do you know that we are made up of atoms? Do you know we are made up of cells? Do you know that we are on a planet orbiting a sun that is part of a galaxy? Or maybe the world is supported on the backs of giant elephants that stand on the shell of an enormous turtle?In science, unlike in polite conversation, we are quite accustomed to questioning assumptions and the question "Why?" can usually be translated as "How do you know?" [Do be warned that in typical social settings, many people do not take well to having their assumptions directly questioned, so tread carefully.]Up to the end of the 19th Century, we knew that matter was made up of atoms (seemingly indivisible lumps) but no one was able to show that these atoms had internal structure and inner parts.Of course, so much has changed since then -- visible technologies like computers and robots powered fundamentally by our sophisticated understanding of science and mathematics. Not to forget two World Wars, several pandemics including COVID-19, a sobering global stockpile of weapons of mass destruction... but it is always up to us to shape the future.
Dalton's indivisible spheres
When we look around us, everything seems so different. Burning wood turns into fire and ash. To think that oxygen is actually reacting with carbon!John Dalton's breakthrough was realising that so many of the complex chemical processes were fundamentally based on rearrangements of unchanging pieces.Unlike early philosophers like Democritus who imagined and argued for indivisible particle through the impossibility of splitting a chunk of matter indefinitely, Dalton was able to gather robust experimental evidence to support his claims. He focused on experiments with gases and discovered the law of partial pressures: that the combined pressure of a mixture of gases was given by the sum of the partial pressures that each individual gas exerted while occupying the same space.By carefully measuring the relative mass of gases like water vapour, ammonia, and carbon dioxide, in 1803, Dalton’s proposed this atomic model:- Gases are made up of small particles, which are referred to as atoms.
- All atoms of a given element are identical.
- The atoms of different elements vary by mass and other properties.
Building on this work, he and other scientists refined and extended the model, leading to a chart and organisation of atoms, molecules, elements, compounds, and so on. The evidence from further experiments suggested that atoms are indestructible, with chemical reactions resulting in their rearrangement, but not their creation or destruction.Thomson's discovery of electrons
Thomson's discovery of electrons and the plum-pudding model
Until 1897, atoms were believed to be the smallest units of matter.When experimenting with the phenomenon of "cathode rays", J. J. Thomson discovered that these rays were made of charged particles (we call them electrons) because they could be deflected by electric and magnetic fields.He proposed that these cathode rays were ejected from atoms, and that atoms are normally electrically neutral with equal amounts of positive and negative charge. When the atoms eject electrons, they become charged ions.Watch the video below to find out more about Thomson's discovery of electrons, and the delicious-sounding plum-pudding model of the atom.Thomson's Plum Pudding Model of the Atom by Veritasium -
Deflection of an electron beam by a magnetic field
J. J. Thomson used a cathode ray tube similar to what is shown in the video below. He observed that the beam was deflected by both magnetic and electric fields. This allowed him to measure the mass-to-charge ratio of the particles in the beam, which did not depend on the atoms producing the beams.Watch the video and admire the beauty of electron beams. You may wish to use Fleming's left-hand rule (or the right-hand grip convention for the Lorentz force) to analyse the magnetic deflection of the electron for yourself. [Click here if necessary to revise Force on a Moving Charge in a Magnetic Field.] -
-
Rutherford's discovery of the atomic nucleus
Rutherford's discovery
In 1909, Ernest Rutherford, a former student of J. J. Thomson, realised that there was something very dense buried deep within the plum-pudding, which meant that it was not so appropriate to think of it as a plum-pudding anymore. Rather, it seemed like the atom was a microcosm of the solar system!The experiment goes like this...- Prepare some thin gold foil.
- Bombard helium-4 ions (also known as alpha particles) at the foil.
- Most of the alpha particles pass through with small deflections, totally expected, like bullets going through paper.
- But some of these particles bounce right back!
Rutherford deduced that the atoms must have their mass concentrated in a tiny volume (the "nucleus"), such that most alpha particles do not pass very near these concentrations, but some alpha particles happen to pass very near and this basically results in an elastic collision between a relatively low-mass alpha particle and a relatively high-mass gold nucleus!In other words, in this model of the atom, the nucleus was like our Sun and the electrons (discovered by Thomson) were like orbiting planets. Of course, the attractive force was not gravitational but of electromagnetic origin.Watch the video below to find out more about the observations that led to Rutherford's discovery of the atomic nucleus and the planetary model for the atom.Cathode Rays Lead to Thomson's Model of the Atom by VeritasiumAlpha particle scattering by gold nucleus
In the simulation below, you can fire alpha particles and observe their deflection by the gold foil.Click on "Rutherford Atom" to visualise what Rutherford actually observed, and click on "Plum Pudding Atom" to visualise what Rutherford was expecting to observe.There is a button that you need to press to "turn on" the alpha source. You may wish to click the "Traces" checkbox to visualise the trails of the alpha particles.
Video
[text]
Version:
Other Resources
https://vle.learning.moe.edu.sg/moe-library/lesson/view/b6b61e5a-1523-4889-92af-a3b73e21509a?fromCcpm=false
Frequently Asked Questions about Mass Spectrometry and Atomic Structure
1. What is a mass spectrometer and what is its basic function? A mass spectrometer is an instrument that analyzes the mass-to-charge ratio of charged particles. Its basic function involves ionizing a sample, separating the ions based on their mass-to-charge ratio using magnetic and/or electric fields, and then detecting the relative abundance of each ion. This allows scientists to determine the masses of atoms and molecules within a sample.
2. How does a mass spectrometer work, based on the provided simulation description? The simulation allows users to manipulate various parameters such as the mass, charge, and speed of particles, as well as the strength of the magnetic and electric fields within the spectrometer. By observing how these changes affect the path of the particles, one can understand the fundamental principle: charged particles moving through magnetic and electric fields experience forces that cause them to deflect. The extent of this deflection is determined by their mass-to-charge ratio, allowing for separation and analysis.
3. What can be learned about atomic structure from the principles behind mass spectrometry? The development and application of mass spectrometry have significantly contributed to our understanding of atomic structure. Early experiments, like Thomson's work with cathode rays (which utilized magnetic and electric fields to deflect charged particles), led to the discovery of the electron and the determination of its mass-to-charge ratio. This demonstrated that atoms were not indivisible and had internal components. Mass spectrometry allows for precise measurements of atomic and isotopic masses, providing evidence for the existence of isotopes (atoms of the same element with different numbers of neutrons) and further refining our understanding of the composition of atoms.
4. How did J.J. Thomson's work contribute to our understanding of the atom, and how does it relate to the principles used in a mass spectrometer? J.J. Thomson's experiments with cathode rays demonstrated that atoms contained negatively charged particles (electrons) that could be deflected by electric and magnetic fields. By measuring the deflection, he was able to determine the mass-to-charge ratio of these particles. This was a crucial step in realizing the internal structure of the atom. The principle of using electric and magnetic fields to deflect and analyze charged particles, central to Thomson's work, is also the fundamental basis of how a mass spectrometer separates ions according to their mass-to-charge ratio.
5. How did Rutherford's gold foil experiment change our understanding of the atom, and what was the key finding? Rutherford's gold foil experiment involved firing alpha particles at a thin gold foil. The unexpected result was that a small fraction of these particles were deflected at large angles, some even bouncing back. This led Rutherford to conclude that the atom has a small, dense, positively charged nucleus at its center, where most of its mass is concentrated. This replaced the earlier "plum-pudding" model and proposed a more "planetary" model of the atom with electrons orbiting the nucleus. While not directly a mass spectrometry technique, Rutherford's discovery of the nucleus provided the foundation for understanding the source of mass and charge within an atom, which are the key properties analyzed by a mass spectrometer.
6. The text mentions Dalton's atomic model. How does it differ from the later models by Thomson and Rutherford, and what were Dalton's main contributions? Dalton's atomic model proposed that elements are made of indivisible particles called atoms, all atoms of a given element are identical, and chemical reactions involve the rearrangement of these indestructible atoms. His main contributions were providing experimental evidence for the atomic theory and establishing concepts like elements, compounds, and chemical reactions as rearrangements of atoms. This model differed from Thomson's and Rutherford's by not recognizing the internal structure of the atom or the existence of subatomic particles like electrons and the nucleus. Thomson showed atoms had negatively charged parts, and Rutherford revealed the atom had a dense, positively charged core.
7. For whom is the mass spectrometer simulation intended, and what are some of the learning goals associated with it? The mass spectrometer simulation is intended for students, particularly those at the pre-university level (H2 Physics syllabus). The sample learning goals indicate that it is designed to help students understand the principles of electromagnetism as applied to charged particles and potentially to infer concepts related to atomic structure, although the specific syllabus topic mentioned (Rutherford scattering) is from a different simulation. Generally, such a simulation would aim to help students visualize how manipulating variables like mass, charge, speed, and field strengths affects the motion of ions in a mass spectrometer.
8. Beyond mass spectrometry, what broader themes about the nature of scientific inquiry and the evolution of scientific models are highlighted in the provided text? The text emphasizes the importance of questioning assumptions ("How do you know?"), the role of experimental evidence in building scientific understanding (contrasting Democritus' philosophical arguments with Dalton's experimental data), and the iterative nature of scientific progress. The progression from Dalton's indivisible atoms to Thomson's plum-pudding model and then to Rutherford's nuclear model illustrates how scientific models evolve as new evidence emerges and challenges existing ideas. The text also implicitly highlights the connection between fundamental scientific discoveries (like the structure of the atom) and the development of technologies (like computers) and even significant global events.
- Details
- Written by Siti
- Parent Category: 02 Static Electricity
- Category: 01 Electromagnetism
- Hits: 11502








.png
)


