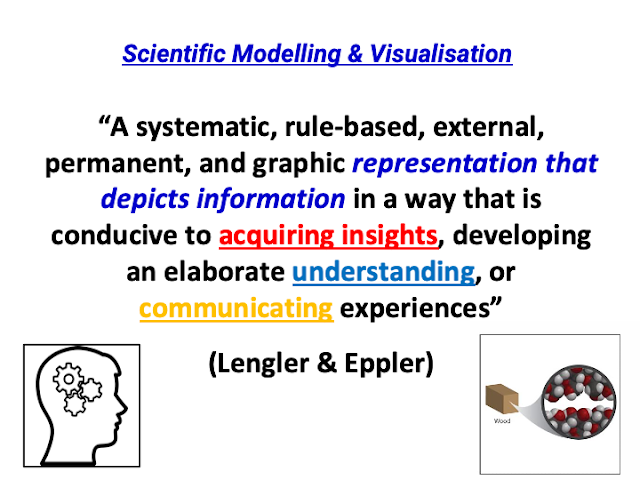
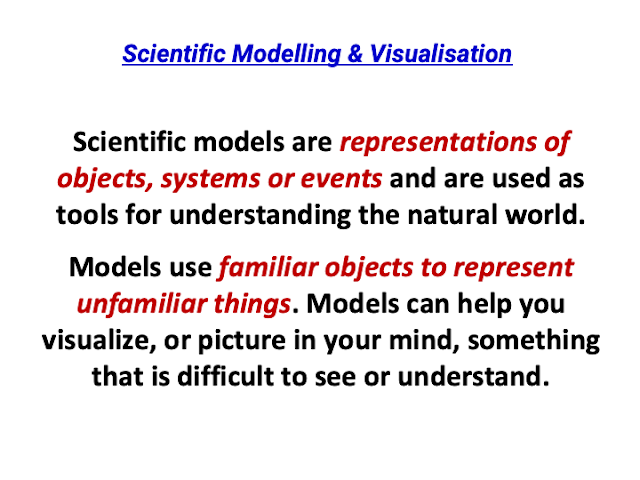
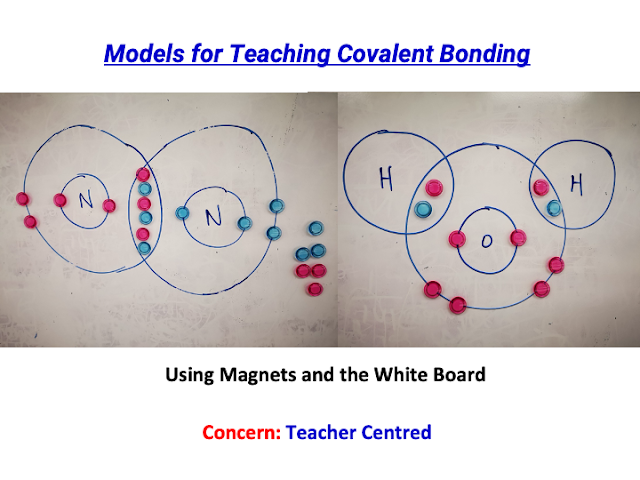
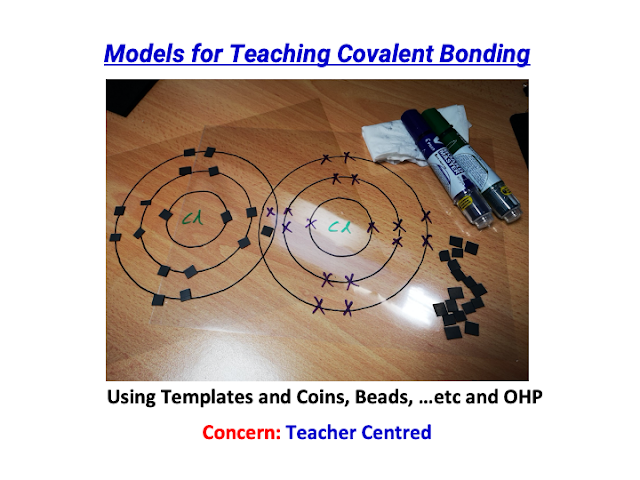
Executive Summary:
This document summarizes a sharing session by David Loh focused on a newly developed interactive simulation for teaching dot and cross diagrams for covalent bonding. The primary theme revolves around the positive reception and perceived pedagogical benefits of this open educational resource among chemistry teachers. Key aspects highlighted include student engagement, self-directed learning, immediate feedback, ease of use, and the potential for enhancing understanding of valence electrons and polyatomic ions. Teachers also provided valuable feedback and suggestions for future improvements, such as including ionic bonding, macromolecules, options for deducing valence electrons, and addressing technical issues.
Main Themes and Important Ideas/Facts:
- Development and Focus of the Simulation:
- The sharing centered on a JavaScript simulation applet designed to help secondary school chemistry students learn about covalent bonding through the creation of dot and cross diagrams.
- The applet is accessible online via the Open Educational Resources / Open Source Physics @ Singapore platform.
- A key feature is its interactivity, allowing students to manipulate electrons to form bonds.
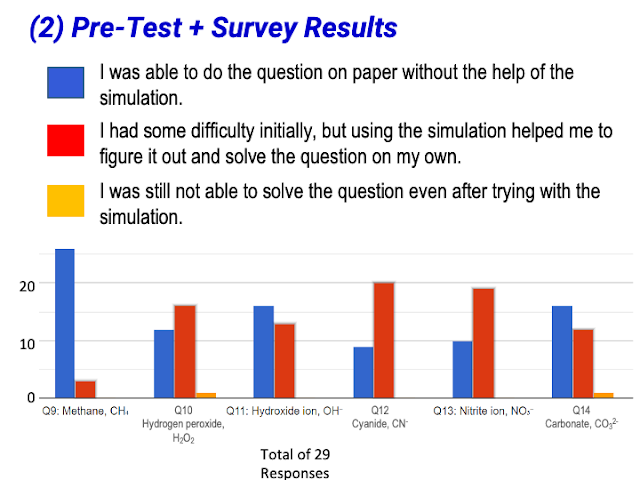
- The simulation includes examples of polyatomic ions.
- Positive Teacher Feedback and Perceived Benefits:
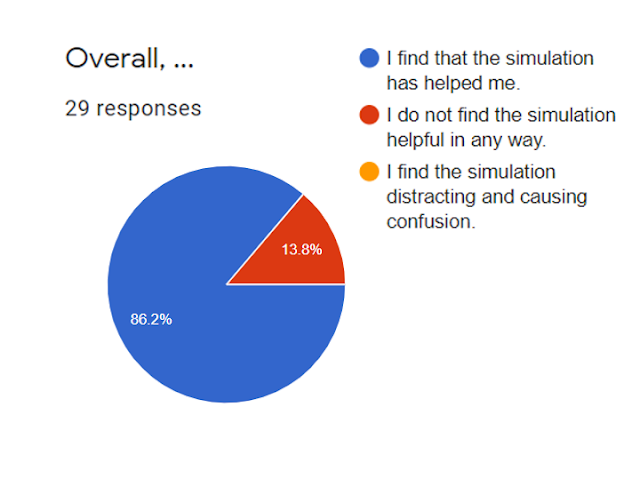
- The overwhelming response from teachers who used or reviewed the simulation was positive.
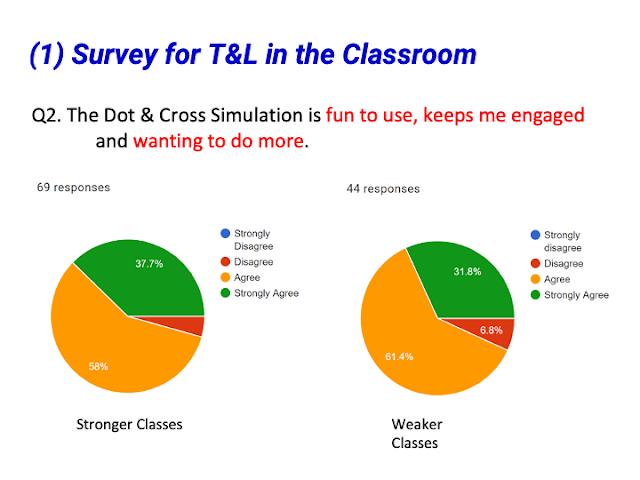
- Increased Student Engagement: Many teachers noted that the simulation was more engaging for students compared to traditional teaching methods.
- Quote: "Engaging for students as compared to current teaching methods."
- Quote: "Engaging for students, allows students to learn independently"
- Promotion of Self-Directed Learning: The interactive nature allows students to explore bonding concepts independently.
- Quote: "This promotes self-directed learning."
- Quote: "It can help students in their self-directed learning."
- Quote: "That the learning experience is different from a regular chalk-and-talk in class, students can be more self-directed."
- Quote: "Allows students to explore to discover their own learning."
- Quote: "Very user friendly, creates opportunity for self exploration and can learn the concept from mistakes."
- Quote: "Students can learn at their own pace."
- Instant Feedback and Error Correction: The simulation provides immediate feedback, allowing students to identify and correct mistakes.
- Quote: "Instant feedback, can embed in sls"
- Quote: "Hands on and students can obtain immediate feedback for their answer ."
- Quote: "Immediate feedback"
- Quote: "It offers feedback to the students and let them explore."
- Quote: "The feedback given when answer is wrong"
- Quote: "It is good for them to see whether the student are making mistakes or not."
- Quote: "Instant feedback and subtle scaffolds built-in."
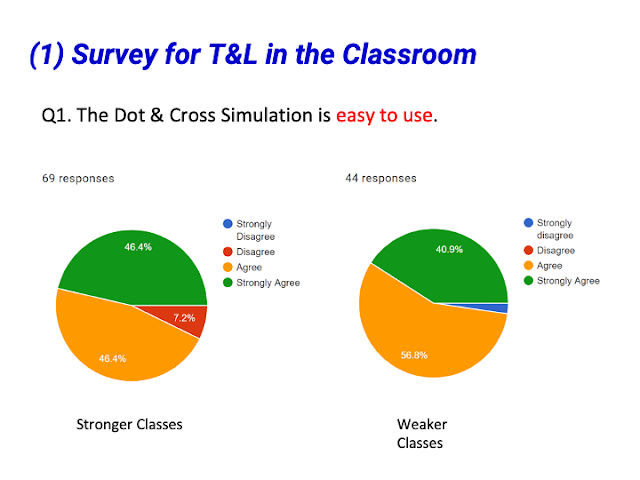
- Ease of Use and Accessibility: Teachers found the simulation user-friendly and easily accessible online.
- Quote: "Easy to use."
- Quote: "simple to use"
- Quote: "Easy to manipulate."
- Quote: "I like that it is online. can easily accessible."
- Quote: "very user friendly"
- Quote: "I think it is very easy for students to use."
- Quote: "User friendly and not complicated with too many functions"
- Quote: "Easy and simple to use. Very visual and interactive for students to learn dot and cross diagram."
- Quote: "Easy to use"
- Quote: "Interactive and intuitive"
- Quote: "User friendly, easy to embed, easily available for students"
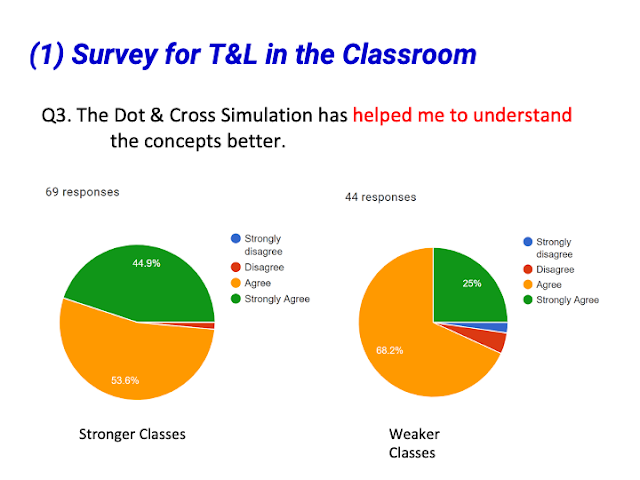
- Enhanced Conceptual Understanding: The simulation helps students understand the concept of valence electrons and how they are involved in bonding.
- Quote: "I like that it gives students the opportunity to explore the bonding on their own and to allow students to understand the concept of the number of valence electrons that each element has."
- Quote: "Students can explore on their own and understand covalent bonding better"
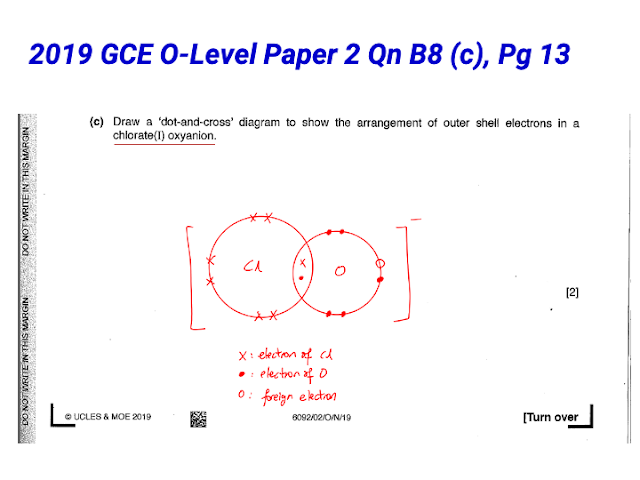
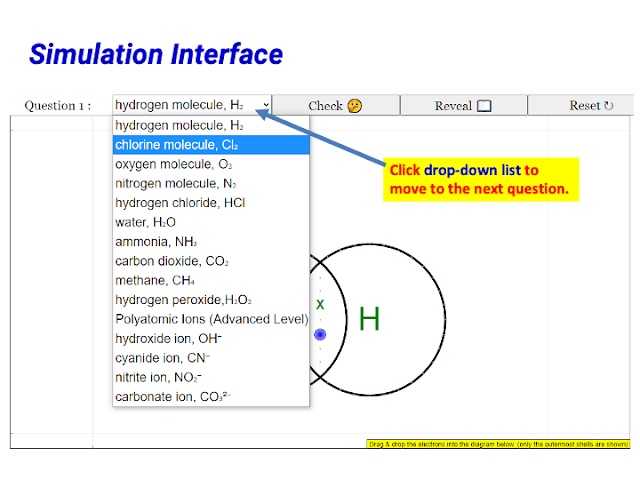
- Support for Polyatomic Ions: The inclusion of polyatomic ions was seen as beneficial for higher-order thinking.
- Quote: "It makes it easy for pure chemistry students to realise that foreign electrons are involved in polyatomic ions."
- Quote: "It includes polyatomic ions which is often promotes higher order thinking. It also give immediate feedback to students' answer"
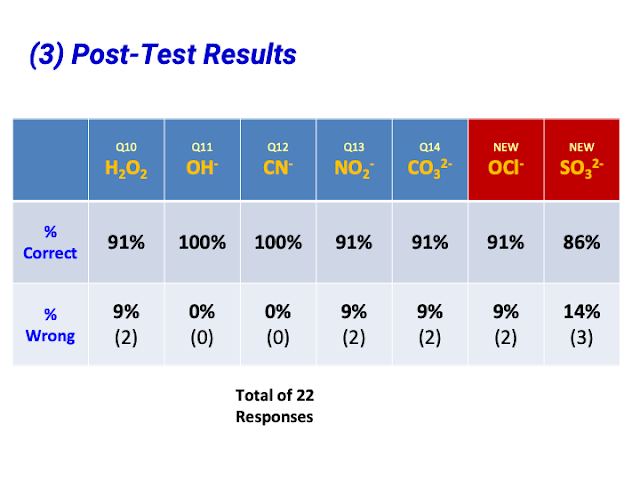
- Assessment Potential: Teachers appreciated the potential for post-activity tests and even converting the simulation into quizzes.
- Quote: "And I really like that we do a post activity test to check the students learning."
- Quote: "Converted to quiz with marks"
- Flexibility in Accepted Answers: The simulation showed some flexibility in accepting paired electrons even if not in a specific arrangement.
- Quote: "Flexibility allowed - even though electrons are paired, still accepted as good answers."
- Sensory Engagement: One teacher noted the ability to judge mastery level by listening to the sound of student responses on their devices.
- Quote: "Concurrently hands on try by all students - can listen to the sound of their responses in the IT gadgets to judge mastery level"
- Teacher Suggestions and Areas for Improvement:
- Inclusion of Ionic Bonding and Macromolecules: Several teachers requested the inclusion of ionic bonding and macromolecules to make the simulation more comprehensive, especially for pure chemistry students.
- Quote: "Looking forward to the ionic bonding version."
- Quote: "To include ionic bonding, marcromolecules to cover for pure chemistry students as well."
- Quote: "Include ionic bonding"
- Quote: "Good that u r working on ionic bond."
- Scaffolding for Weaker Students: A suggestion was made to include questions where students complete diagrams for individual atoms to scaffold learning.
- Quote: "Is it possible to have questions for students to complete diagrams for atoms so that they serve to scaffold learning for weaker students or lower sec students?"
- Deducing Valence Electrons: Some teachers suggested providing only the proton number and requiring students to deduce the number of valence electrons.
- Quote: "Include portion where student need to deduce the number of valence electron"
- Quote: "Perhaps can just give proton number instead of valence electron so can make them find out no of valence electrons"
- Quote: "Providing information such as periodic table / element only for students to decide on the electronic configurations / valence electrons."
- Technical Issues: One teacher reported difficulty dragging electrons due to sensitivity.
- Quote: "Sometime the electrons are difficult to drag, as in it is not very sensitive. For example, I wanted to drag a blue electron but I keep dragging the green electron that is below it instead"
- Mobile Device Compatibility: A request was made for improved usability on mobile phones.
- Quote: "i hope it can be used on a handphone easily :)"
- Embedding in Learning Management Systems: Teachers inquired about the possibility of embedding the simulation in platforms like SLS (Student Learning Space).
- Quote: "Can this be embedded in sls"
- Quote: "Instant feedback, can embed in sls"
- Student Arrangement of Atoms: A suggestion was made to allow students to arrange atoms themselves in some molecules (e.g., H₂O₂, carbonate ion) rather than having them pre-arranged.
- Quote: "The atoms have already been pre-arranged for the students. I thought it would be good for them to arrange on their own for some of the molecules like H2O2"
- Quote: "It will be good if the simulation does not have the circle already drawn. Coz sometimes students have problem knowing how the electrons are shared. E.g. carbonate ion, might not know carbon is in the middle. Or hydrogen peroxide where oxygen should be between the 2 hydrogen."
- Lagging Issues: One user noted the simulation being slightly laggy.
- Quote: "it is a little laggy."
- Option to Remove Circles: A suggestion for A-level teaching was to have an option to turn on/off the circles representing electron shells, as they are not typically used at that level.
- Quote: "Won't use this simulation as at the 'A' levels, we don't draw circles for dot-cross diagrams. Will be good if there could be an option to click to turn on /off the circles. Thanks."
- More Precise Electron Pairing: A suggestion to ensure correct answers require appropriate pairing of electrons, not just the correct number.
- Quote: "will perhaps be good if the correct answer will be accepted if pairing of electrons are done accordingly instead of being in a random arrangement"
- Visualization of Inner Electrons: A request to include inner electrons to illustrate the concept of fully filled shells.
- Quote: "What about drawing the inner electrons too? So students will be able to understand there's not just valence electrons but also the concept of fully filled inner electron shells."
- Backend Data Collection: A suggestion for a CMS to track student mistakes and scores.
- Quote: "If there is a backend CMS that captures students’ common mistakes , scores etc , would be great"
- Explanation for Polyatomic Ions: A question on how to better explain polyatomic ions using the simulation.
- Quote: "For polyatomic ions, is there any way to explain to student?"
- Free Movement of Electrons: A suggestion to allow electrons to be freely moved without predefined locations.
- Quote: "Good if the electrons can be free to move"
- Advanced Simulation Features: Requests for simulations showing all electrons (not just valence), drag-and-drop electron shells, and addressing the lack of 3D visualization.
- Quote: "1) Really hope that creator can create the simulation for students to show all electrons for ionic bonding / covalent bonding instead of just valence electrons. 2) Suggestion for students to drag and drop electron shells / brackets instead of providing it in the simulation as it give students hints the substances are bonded in certain manner. 3) Providing information such as periodic table / element only for students to decide on the electronic configurations / valence electrons. 4) The dot-and-cross simulation wont be able to provide the 3D arrangement for students to visualize how some of these molecules looks like."
- Related Resources and Context:
- The page also lists numerous other interactive resources, primarily focused on physics, developed under the Open Educational Resources / Open Source Physics @ Singapore initiative.
- These resources cover a wide range of topics and utilize HTML5 and JavaScript.
- The platform emphasizes the use of open educational resources and encourages sharing among educators.
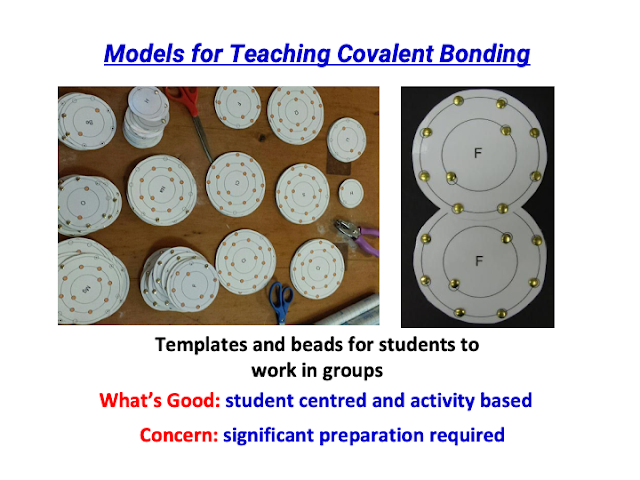
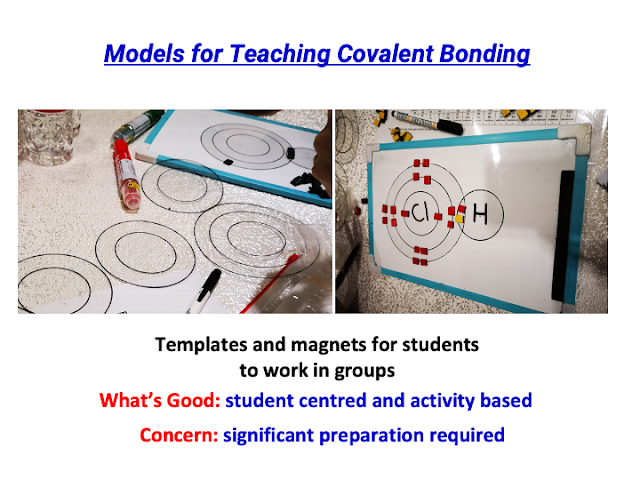
- The mention of a physical tactile version using laminated transparency cards for covalent bonding visualization suggests a multi-modal approach to teaching.
Conclusion:
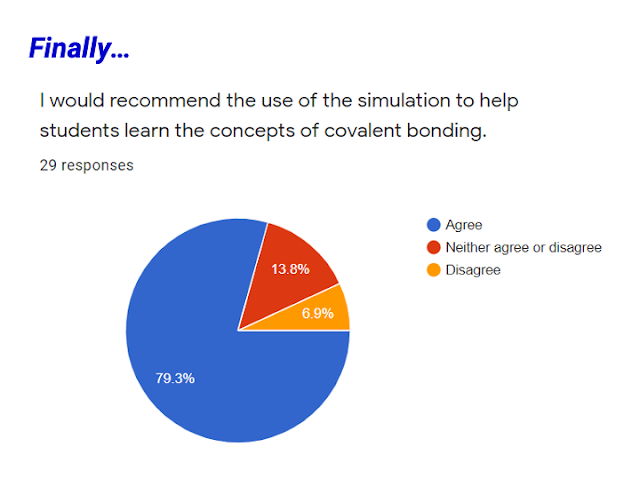
David Loh's sharing session highlights a valuable and well-received interactive simulation for teaching covalent dot and cross diagrams. The positive feedback from teachers underscores the potential of such tools to enhance student engagement, promote self-directed learning, and provide immediate feedback in chemistry education. The constructive suggestions for improvement offer a roadmap for further development, aiming to create an even more comprehensive and effective resource for teaching chemical bonding concepts.
Chemistry Learning Day C3 Sharing Review
Study Guide
This study guide is designed to help you review the key concepts and takeaways from the "20200819 AST Chemistry Chapter Chemistry Learning Day C3 sharing by David Loh" resource. It focuses on the feedback provided by teachers on the dot-and-cross diagram simulation tool.
Key Areas to Focus On:
- Purpose and Subject Matter: Understand the chemistry topic addressed by the shared material.
- Simulation Tool: Identify the core resource being discussed and its function.
- Teacher Feedback (Positive Aspects): Recognize the reasons why teachers found the simulation beneficial.
- Teacher Feedback (Suggestions for Improvement): Understand the areas where teachers proposed enhancements or identified limitations.
- Target Audience: Determine the educational levels and types of chemistry students for whom this resource is intended.
- Pedagogical Implications: Consider how this type of interactive tool can impact teaching and learning in chemistry.
Review Strategies:
- Skim the entire document: Get a general overview of the content and the flow of information.
- Focus on the headings: Pay close attention to the main sections: "Breadcrumbs," the title, "What I like about the simulation?," and "Other Feedback, Suggestions or Areas for Improvement."
- Identify recurring themes: As you read the teacher feedback, note down any ideas or opinions that are mentioned multiple times. Categorize these into positive aspects and suggestions for improvement.
- Consider the context: Remember that this is a sharing session about a specific tool. Think about why teachers might find certain features helpful or suggest particular changes in the context of their classroom teaching.
- Reflect on the broader implications: How does the feedback on this specific simulation relate to the use of interactive resources in science education in general?
Quiz
Answer the following questions briefly in 2-3 sentences each.
- What specific chemistry topic is the simulation tool primarily designed to help students understand, based on the "Secondary" and "Chemistry" breadcrumbs?
- According to the teacher feedback, what is a key advantage of using this simulation compared to traditional teaching methods?
- Several teachers mentioned "self-directed learning." Explain in your own words how this simulation promotes self-directed learning among students.
- What kind of immediate benefit do students receive when using the simulation, as highlighted by multiple teachers?
- What did some teachers suggest regarding the level of scaffolding provided in the simulation, particularly for weaker or younger students?
- A few teachers mentioned the possibility of embedding the simulation in "sls." What is likely meant by "sls" in this educational context?
- What technical issues or limitations with the simulation were brought up by some of the teachers in their feedback?
- Several teachers expressed interest in future versions of the simulation. What specific enhancements related to different types of chemical bonding were suggested?
- What features of the simulation did teachers praise in terms of its ease of use for both themselves and their students?
- One teacher mentioned that at the 'A' levels, they don't draw circles for dot-cross diagrams. What suggestion did they make based on this observation?
Quiz Answer Key
- The simulation tool is primarily designed to help students understand the particulate nature of matter, specifically focusing on atomic structure and stoichiometry, as indicated by the "Atomic Structure and Stoichiometry" breadcrumb.
- A key advantage of using this simulation, according to teacher feedback, is that it is more engaging for students compared to current teaching methods and allows for a different learning experience than traditional chalk-and-talk.
- The simulation promotes self-directed learning by allowing students to explore chemical bonding concepts independently, experiment with electron arrangements, and receive immediate feedback on their attempts, encouraging them to learn from their mistakes at their own pace.
- Students receive the immediate benefit of instant feedback on their answers, which helps them to quickly identify errors and understand the correct way to represent chemical bonds using dot-and-cross diagrams.
- Some teachers suggested including questions for students to complete diagrams for individual atoms first, which would serve to scaffold learning for weaker or lower secondary students before they tackle molecule formation.
- "sls" likely refers to the Student Learning Space, a national online learning platform used in Singaporean schools, suggesting that teachers would like to integrate this interactive tool into their existing digital learning environment.
- Some technical issues mentioned included difficulty in dragging electrons (sensitivity issues) and occasional lag. One teacher also questioned if it could be converted to HTML5 for better compatibility and suggested an option to turn circles around the atoms on or off.
- Several teachers expressed interest in the development of simulations for ionic bonding and macromolecules to further support the learning of pure chemistry students beyond just covalent bonding.
- Teachers consistently praised the simulation for being easy and simple to use, user-friendly, and not complicated with too many functions, making it accessible for both educators and students.
- The teacher suggested that it would be good to have an option to click and turn on or off the circles used in the dot-cross diagrams since circles are not typically used at the 'A' levels, catering to the needs of more advanced students.
Essay Format Questions
- Analyze the teacher feedback provided in the document. What are the most significant strengths of the dot-and-cross diagram simulation tool according to educators? Support your answer with specific examples from the text.
- Based on the suggestions for improvement, what are the potential limitations or areas where the current dot-and-cross diagram simulation could be enhanced to better serve the needs of chemistry students and teachers?
- Discuss the pedagogical benefits of using interactive simulations like the one described in the document for teaching abstract concepts in chemistry, such as chemical bonding. Use evidence from the teacher feedback to support your arguments.
- Considering the feedback provided, how well does the dot-and-cross diagram simulation cater to diverse learners in a chemistry classroom? What modifications or additions could be made to increase its accessibility and effectiveness for all students?
- Evaluate the overall value and potential impact of open educational resources like the dot-and-cross diagram simulation on chemistry education, based on the teacher responses and the nature of the resource itself.
Glossary of Key Terms
- Dot-and-Cross Diagram: A visual representation of the valence electrons in an atom or molecule, used to show how electrons are shared or transferred during chemical bonding. Dots and crosses are used to distinguish between the electrons originating from different atoms.
- Valence Electrons: The electrons in the outermost shell of an atom that are involved in forming chemical bonds.
- Ionic Bonding: A type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, typically formed when electrons are transferred from one atom to another.
- Covalent Bonding: A type of chemical bonding that involves the sharing of electron pairs between atoms.
- Polyatomic Ions: Ions composed of two or more atoms covalently bonded together that carry an overall electric charge.
- Self-Directed Learning: An educational approach where students take the initiative and responsibility for their own learning, including setting goals, identifying resources, and evaluating their progress.
- Scaffolding (in education): Providing temporary support or guidance to learners that is gradually withdrawn as they become more competent.
- User-Friendly: Designed with ease of use in mind, making it simple for individuals to understand and operate.
- UI (User Interface): The means by which a user interacts with a computer program or electronic device, including menus, buttons, and other visual elements.
- HTML5: The latest evolution of the standard that defines HTML (Hypertext Markup Language), used for structuring and presenting content on the World Wide Web. It supports multimedia and interactive elements.
- SLS (Student Learning Space): A national online learning platform used in Singapore to support teaching and learning.
- Macromolecules: Very large molecules, such as polymers or proteins, typically formed by the polymerization of smaller subunits.
- CMS (Content Management System): A software application or set of related programs that are used to create and manage digital content.
- Pedagogical: Relating to teaching or education.
awesome sharing by David!
enjoy!
 |
| video is private, sorry! |
 |
| https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
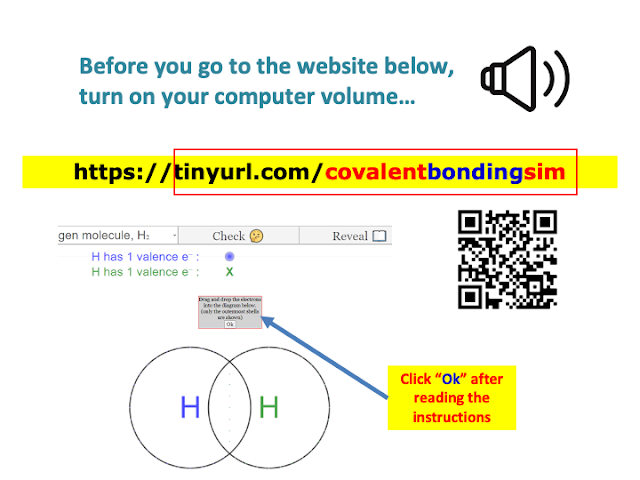 |
| https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| results https://docs.google.com/forms/d/1qF5CuDpEol4pypxJtz9eTGdajF_pI_yGJxuoi0i1U8o/viewanalytics |
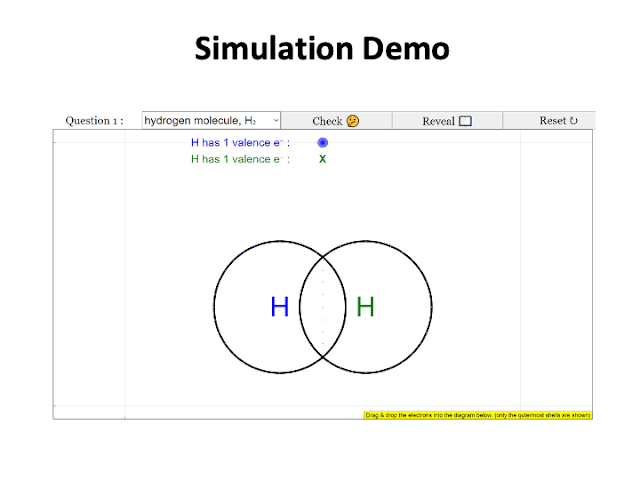
What I like about the simulation?
- Engaging for students as compared to current teaching methods. Looking forward to the ionic bonding version. I will try it in the next fortnight with both Sec 3 Express and Sec 4 Express students
- Self explanatory.. a variety of answers accepted.
- Student centered especially to higher ability students
- Instant feedback, can embed in sls
- I like that it gives students the opportunity to explore the bonding on their own and to allow students to understand the concept of the number of valence electrons that each element has.
- Easy to use. And I really like that we do a post activity test to check the students learning.
- This promotes self-directed learning.
- Engaging for students, allows students to learn independently
- Hands on and students can obtain immediate feedback for their answer .
- simple to use
- It can help students in their self-directed learning.
- Immediate feedback
- That the learning experience is different from a regular chalk-and-talk in class, students can be more self-directed.
- Self directed, good for revision as well
- It’s user friendly and the explanation given is clear too
- Easy to manipulate.
- I like that it is online. can easily accessible.
- Good for beginner students as the structure is there. They just need to figure out where the electrons go.
- very user friendly
- I think it is very easy for students to use.
- Flexibility allowed - even though electrons are paired, still accepted as good answers.
- It offers feedback to the students and let them explore.
- Concurrently hands on try by all students - can listen to the sound of their responses in the IT gadgets to judge mastery level
- User friendly and not complicated with too many functions
- Easy and simple to use. Very visual and interactive for students to learn dot and cross diagram.
- The feedback given when answer is wrong
- Easy to use
- Interactive
- It makes it easy for pure chemistry students to realise that foreign electrons are involved in polyatomic ions.
- Students can explore on their own and understand covalent bonding better
- Easy to use, clean UI
- User friendly, fuss free
- Very user friendly, creates opportunity for self exploration and can learn the concept from mistakes.
- It is good for them to see whether the student are making mistakes or not.
- Interactive and intuitive
- Instant feedback and subtle scaffolds built-in.
- Easy for students to visualise and manipulate to get the bonding right.
- User friendly, easy to embed, easily available for students and it is really extraordinary effort by David and team to work on this!
- It includes polyatomic ions which is often promotes higher order thinking. It also give immediate feedback to students' answer
- Students can learn at their own pace.
Other Feedback, Suggestions or Areas for Improvement:
- Nil
- Did a physical (tactile) version using laminated transparency cards for T&L of Covalent Bonding in visualisation: https://drive.google.com/file/d/1yDFKTuRrquUSNQEGXAYbVHKB1m_3wODD/view?usp=sharing
- so far so good! :)
- Is it possible to have questions for students to complete diagrams for atoms so that they serve to scaffold learning for weaker students or lower sec students?
- Converted to quiz with marks
- Not at the moment (:
- thank you so much.
- Include portion where student need to deduce the number of valence electron
- Thank u so much for the sharing!
- Perhaps can just give proton number instead of valence electron so can make them find out no of valence electrons
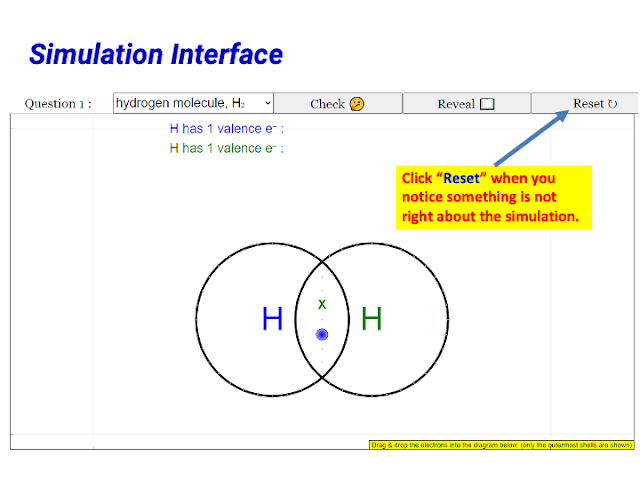
- Sometime the electrons are difficult to drag, as in it is not very sensitive. For example, I wanted to drag a blue electron but I keep dragging the green electron that is below it instead
- -
- i hope it can be used on a handphone easily :)
- Can this be embedded in sls
- The atoms have already been pre-arranged for the students. I thought it would be good for them to arrange on their own for some of the molecules like H2O2
- it is a little laggy. Also, possible to be HTML5? Won't use this simulation as at the 'A' levels, we don't draw circles for dot-cross diagrams. Will be good if there could be an option to click to turn on /off the circles. Thanks.
- will perhaps be good if the correct answer will be accepted if pairing of electrons are done accordingly instead of being in a random arrangement
- To include ionic bonding, marcromolecules to cover for pure chemistry students as well.
- Thank you !
- Thanks for the great simulation!
- What about drawing the inner electrons too? So students will be able to understand there's not just valence electrons but also the concept of fully filled inner electron shells.
- Include ionic bonding
- Allows students to explore to discover their own learning.
- Great simulation
- Good that u r working on ionic bond.
- If there is a backend CMS that captures students’ common mistakes , scores etc , would be great
- Na
- For polyatomic ions, is there any way to explain to student?
- Good if the electrons can be free to move
- 1) Really hope that creator can create the simulation for students to show all electrons for ionic bonding / covalent bonding instead of just valence electrons. 2) Suggestion for students to drag and drop electron shells / brackets instead of providing it in the simulation as it give students hints the substances are bonded in certain manner. 3) Providing information such as periodic table / element only for students to decide on the electronic configurations / valence electrons. 4) The dot-and-cross simulation wont be able to provide the 3D arrangement for students to visualize how some of these molecules looks like.
- It will be good if the simulation does not have the circle already drawn. Coz sometimes students have problem knowing how the electrons are shared. E.g. carbonate ion, might not know carbon is in the middle. Or hydrogen peroxide where oxygen should be between the 2 hydrogen.
Frequently Asked Questions about the Dot and Cross Diagram Simulation
Q1: What is the purpose of the Dot and Cross Diagram simulation?
The primary purpose of the Dot and Cross Diagram simulation, as indicated by teacher feedback, is to provide an engaging and interactive way for students to learn and understand chemical bonding, specifically covalent bonding, at the secondary school level. It allows students to visually construct dot and cross diagrams for molecules, reinforcing their understanding of valence electrons and how atoms share electrons to form bonds.
Q2: How does this simulation enhance student learning compared to traditional teaching methods?
Teachers find the simulation more engaging than traditional chalk-and-talk methods. It promotes student-centered learning, especially for higher ability students, by allowing them to explore bonding concepts independently. The instant feedback mechanism helps students identify and correct their mistakes in real time, fostering a deeper understanding.
Q3: What are the key features that teachers appreciate most about the simulation?
Teachers highlighted several key features they appreciate: its ease of use, clear explanations, the provision of instant feedback, its potential for self-directed learning and revision, and its accessibility online. They also liked the inclusion of polyatomic ions, which encourages higher-order thinking, and the flexibility in accepting correctly paired electrons even if not in a specific arrangement.
Q4: For which levels or types of chemistry students is this simulation most suitable?
Based on the feedback, the simulation is suitable for secondary school chemistry students, including Sec 3 Express and Sec 4 Express levels. It is also considered good for beginner students as the basic structure is provided, allowing them to focus on placing the valence electrons correctly. Some teachers also suggested its potential use for lower secondary students if modified with additional scaffolding.
Q5: What suggestions have teachers provided for improving the simulation?
Teachers offered several suggestions for improvement, including: allowing students to deduce the number of valence electrons instead of being given them directly, improving the drag-and-drop sensitivity of electrons, making it easily usable on mobile phones, allowing students to arrange atoms in molecules themselves (e.g., H2O2), offering an option to turn circles on/off for more advanced students, ensuring correct pairing of electrons is consistently accepted, including content on ionic bonding and macromolecules for pure chemistry students, visualizing inner electrons, creating a backend system to track student progress and common mistakes, allowing freer movement of electrons, and providing options to build electron shells or brackets from scratch.
Q6: Can this simulation be integrated with existing learning management systems?
Yes, multiple teachers specifically asked if the simulation could be embedded in their school's Student Learning Space (SLS), indicating a desire for seamless integration into their existing online teaching platforms.
Q7: Does the simulation address the drawing of dot and cross diagrams for polyatomic ions?
Yes, the current version of the simulation includes the drawing of dot and cross diagrams for polyatomic ions. Teachers specifically mentioned that this feature promotes higher-order thinking in students.
Q8: Where can this simulation be found and are there other related resources available?
The specific link to the Dot and Cross Diagram simulation is provided as https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8. This simulation is part of the Open Educational Resources / Open Source Physics @ Singapore project, which offers a wide range of interactive resources for physics and chemistry. The platform also includes other simulations, applets, and learning materials, as evidenced by the extensive list of resources provided in the source document.