https://lab.concord.org/embeddable.html#interactives/sam/diffusion/1-dropping-dye-on-click.json
Overview
This briefing document reviews the "Experiments on Diffusion" resource provided by the Concord Consortium and hosted on the Open Educational Resources / Open Source Physics @ Singapore platform. This resource consists of interactive simulations designed to help secondary school learners understand the process of diffusion and the factors that influence its rate. The document highlights the main themes, learning goals, and the broader context of this resource within the platform.
2. Main Themes and Key Ideas
The primary theme of this resource is the scientific concept of diffusion. The simulations aim to make this abstract concept more tangible and explorable for students. Key ideas presented through the simulations include:
- Diffusion as Continuous Random Motion: The first simulation focuses on visualizing the movement of dye particles within water particles. It is intended to guide learners to understand that diffusion occurs due to the constant, random movement of particles. As stated in the description, learners can "explain how diffusion occurs through a series of continuous random motion." This aligns with the kinetic theory of matter.
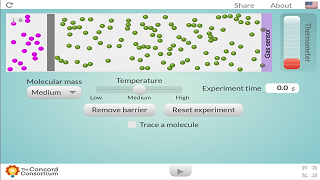
- Influence of Molar Mass and Temperature on Diffusion Rate: The second simulation allows learners to investigate how these two factors affect how quickly diffusion occurs. This hands-on, virtual experimentation allows students to discover relationships between these variables and the rate of diffusion. The description explicitly states that "the effects of molar mass and temperature can be used as an exploratory activity for learners to conclude the effects that these have on the rate of diffusion."
- Inquiry-Based Learning: The resource is designed to facilitate an "Inquiry-based Learning activity." This pedagogical approach encourages students to actively explore the simulations, manipulate variables, observe the outcomes, and draw their own conclusions about the underlying scientific principles.
3. Important Facts and Details
- Target Audience: The resource is specifically designed for secondary level learners.
- Simulation Components: The resource includes two distinct simulations:
- One demonstrating the basic process of diffusion at a particle level.
- Another allowing the manipulation of molar mass and temperature to observe their effects on the diffusion rate.
- Learning Goals: The stated Sample Learning Goals indicate that the primary objective is for students to "experiment on diffusion, manipulating temperature and molar mass." This emphasizes active engagement and data-driven understanding.
- Resource Type: This is an interactive simulation, likely utilizing JavaScript and HTML5, as indicated by other resources listed on the platform. This format allows for direct manipulation and real-time feedback, enhancing the learning experience.
- Platform Context: The resource is part of a larger collection of "Open Educational Resources / Open Source Physics @ Singapore." This suggests a commitment to providing freely accessible and modifiable educational materials, particularly in the realm of physics. The platform hosts a wide variety of simulations, interactive applets, and games covering various science and mathematics topics.
- Attribution: The Concord Consortium is credited as the creator of this specific resource. The platform itself operates under a "Creative Commons Attribution-Share Alike 4.0 Singapore License," highlighting the open nature of the content.
4. Connections to Other Platform Resources
The provided text includes a long list of other resources available on the Open Educational Resources / Open Source Physics @ Singapore platform. These span a wide range of topics, including:
- Other physics concepts (e.g., convection current, buoyancy, projectile motion, waves, optics, electromagnetism).
- Chemistry topics (e.g., chemical bonding, balancing equations).
- Mathematics (e.g., adding, geometry, algebra, calculus).
- Interactive games for language learning (Malay, Chinese) and other subjects.
- Tools for teachers and assessment design.
The presence of numerous other simulations utilizing JavaScript and HTML5 suggests a consistent technological approach across many of the platform's resources. The various "SLS Hackathon" projects indicate a community engagement aspect, with educators creating and sharing interactive learning tools.
5. Potential Applications and Considerations
- This diffusion simulation can be a valuable tool for educators to visually demonstrate a microscopic process that is often difficult for students to grasp.
- The ability to manipulate variables like molar mass and temperature provides opportunities for students to conduct virtual experiments, collect qualitative data (observing the rate), and develop an intuitive understanding of the factors influencing diffusion.
- The inquiry-based nature encourages critical thinking and scientific reasoning skills.
- Teachers can use this simulation as a standalone activity, as part of a larger lesson on kinetic theory or transport phenomena, or as a pre-lab or post-lab activity.
- The "For Teachers" section (though not detailed in the provided text) likely contains guidance on how to effectively integrate these simulations into instruction.
6. Conclusion
The "Experiments on Diffusion" resource from the Concord Consortium, hosted on the Open Educational Resources / Open Source Physics @ Singapore platform, offers a valuable and interactive way for secondary students to learn about diffusion. By providing engaging simulations that visualize particle motion and allow for the manipulation of key variables, this resource supports inquiry-based learning and helps students develop a deeper understanding of this fundamental scientific concept. Its placement within a broader collection of open educational resources further enhances its potential impact on science education.
Diffusion Study Guide
Overview of Diffusion Simulation
This resource provides two interactive simulations focused on the phenomenon of diffusion. The first simulation allows learners to observe the diffusion of a dye particle through water particles, emphasizing the role of continuous random motion in this process. The second simulation enables users to explore how molar mass and temperature affect the rate of diffusion, encouraging inquiry-based learning and the development of conclusions based on experimentation.
Key Concepts
- Diffusion: The net movement of particles from a region of higher concentration to a region of lower concentration as a result of random motion.
- Random Motion: The chaotic and unpredictable movement of individual particles due to their kinetic energy. In liquids and gases, this movement is constant and in all directions.
- Concentration Gradient: The difference in the concentration of a substance across a distance. Diffusion occurs down the concentration gradient.
- Molar Mass: The mass of one mole of a substance, typically expressed in grams per mole (g/mol).
- Temperature: A measure of the average kinetic energy of the particles in a substance. Higher temperatures mean particles move faster.
- Rate of Diffusion: How quickly diffusion occurs, often measured by the time it takes for a substance to spread out.
Study Questions
- Describe what happens to a dye particle when introduced into water in the first simulation. What does this observation suggest about the movement of water particles?
- Explain how the concept of continuous random motion is related to the process of diffusion.
- In the second simulation, how does changing the molar mass of the diffusing substance affect the rate of diffusion? Provide a possible explanation for this effect.
- In the second simulation, how does changing the temperature of the system affect the rate of diffusion? Provide a possible explanation for this effect.
- What are some real-world examples of diffusion that are not explicitly mentioned in the simulations?
- How does the concentration gradient influence the direction of net movement of particles during diffusion?
- Can diffusion occur in solids? If so, provide an example. If not, explain why.
- What is the relationship between kinetic energy of particles and temperature? How does this relationship influence diffusion?
- How might the size and shape of a diffusing particle (beyond molar mass) affect its rate of diffusion?
- Based on the simulations, summarize the relationship between temperature, molar mass, and the rate of diffusion.
Quiz
- Describe the movement of the dye particle in the first simulation. How does this relate to the movement of the water molecules themselves?
- Explain why diffusion occurs from an area of high concentration to an area of low concentration in terms of random particle motion.
- According to the second simulation, what is the effect of increasing the molar mass of a substance on its rate of diffusion? Provide a brief explanation.
- Based on the second simulation, how does increasing the temperature affect the rate of diffusion? Explain the underlying reason for this change.
- Give one everyday example of diffusion not directly related to a dye in water.
- What is a concentration gradient, and how does it drive the process of diffusion?
- Briefly describe whether diffusion can happen in different states of matter (solid, liquid, gas) and give one example.
- How does the temperature of a substance relate to the kinetic energy of its particles, and how does this affect diffusion?
- Besides molar mass, what other property of a diffusing particle might influence how quickly it spreads?
- Summarize the main conclusions one can draw about the factors affecting the rate of diffusion based on the two simulations provided.
Answer Key for Quiz
- The dye particle moves randomly throughout the water. This suggests that the water molecules are also in constant, random motion, bumping into the dye particle and causing it to move.
- Diffusion occurs because there are more particles in the high concentration area, leading to more collisions moving particles towards the low concentration area than vice versa, resulting in a net movement down the concentration gradient.
- Increasing the molar mass generally decreases the rate of diffusion. This is likely because heavier particles move more slowly at the same temperature due to their greater inertia.
- Increasing the temperature increases the rate of diffusion. This is because higher temperatures mean particles have more kinetic energy and move faster, leading to more frequent and forceful collisions that spread the substance more quickly.
- Examples include the smell of perfume spreading through a room, sugar dissolving in tea, or oxygen moving from the lungs into the bloodstream.
- A concentration gradient is the difference in the amount of a substance between two areas. It drives diffusion because particles naturally move from regions where they are more abundant to regions where they are less abundant due to random motion.
- Diffusion can occur in gases (e.g., mixing of air molecules), liquids (as seen in the simulation), and even solids (though much slower, e.g., metal atoms diffusing within a metallic structure).
- Temperature is a measure of the average kinetic energy of particles; higher temperature means higher kinetic energy and thus faster particle movement. This increased movement leads to a faster rate of diffusion.
- The size and shape of a particle could affect diffusion; larger or more irregularly shaped particles might encounter more resistance or have fewer opportunities for movement compared to smaller, more symmetrical ones.
- The simulations demonstrate that the rate of diffusion is influenced by both molar mass (inversely related) and temperature (directly related). Higher temperatures and lower molar masses tend to lead to faster diffusion rates.
Essay Format Questions
- Discuss the role of random particle motion in the phenomenon of diffusion. Using examples from the provided simulations, explain how this microscopic movement leads to the macroscopic spreading of substances.
- Analyze the effects of molar mass and temperature on the rate of diffusion, as explored in the second simulation. Explain the underlying molecular reasons for these observed relationships and provide real-world examples where these principles are relevant.
- Compare and contrast the two diffusion simulations provided. What specific aspects of diffusion does each simulation highlight, and how do they contribute to a comprehensive understanding of the process?
- Consider the limitations of the provided simulations in fully representing the complexity of diffusion in real-world scenarios. What other factors, not explicitly modeled, can influence the rate and extent of diffusion in different systems?
- Design a hypothetical experiment or simulation to further investigate a factor that you believe would significantly impact the rate of diffusion (beyond molar mass and temperature). Describe your experimental setup or simulation parameters and predict the expected outcomes.
Glossary of Key Terms
- Diffusion: The process by which particles in a gas or liquid spread out from an area of higher concentration to an area of lower concentration over time due to their random motion.
- Concentration: The amount of a particular substance in a given volume.
- Concentration Gradient: The difference in concentration of a substance across a specific distance.
- Kinetic Energy: The energy an object possesses due to its motion. For particles, it is related to their speed.
- Molar Mass: The mass of one mole of a substance, expressed in grams per mole. It reflects the mass of the constituent particles.
- Mole: A unit of amount in chemistry, representing approximately 6.022 x 10^23 entities (such as atoms or molecules).
- Random Motion (Brownian Motion): The erratic, zigzag movement of microscopic particles suspended in a fluid (a liquid or a gas), resulting from their collision with the fast-moving atoms or molecules in the gas or liquid.
- Rate of Diffusion: A measure of how quickly a substance spreads out through diffusion, often quantified by the change in concentration over time or the distance traveled by particles in a given time.
- Temperature: A measure of the average kinetic energy of the particles within a substance. Higher temperature indicates greater average kinetic energy and faster particle movement.
In this first simulation, learners experience the diffusion of a dye particle through other water particles.
Learners can be guided to explain how diffusion occurs through a series of continuous random motion.
In this second simulation, the effects of molar mass and temperature can be used as an exploratory activity for learners to conclude the effects that these have on the rate of diffusion.
About
Simulation of (1) diffusion and (2) effects of molar mass and temperature onm diffusion.
Sample Learning Goals
Inquiry-based Learning activity for students to experiment on diffusion, manipulating temperature and molar mass.
For Teachers
[SIMU_TEACHER]
Software Requirements
[SIMU_SWREQ]
Translation
[text]
Research
[text]
Video
[text]
Credits
Concord Consortium
Version:
[text]
Other Resources
[text]
Frequently Asked Questions about Diffusion Simulations
1. What is diffusion, as demonstrated by the Concord Consortium simulation?
The first simulation allows learners to observe the movement of a dye particle through water particles. It visually demonstrates that diffusion is the process by which particles spread out from an area of higher concentration to an area of lower concentration due to their continuous, random motion.
2. How can the simulations help learners understand the factors affecting the rate of diffusion?
The second simulation is designed as an exploratory activity where learners can manipulate variables such as molar mass and temperature. By observing the changes in the rate of diffusion under different conditions, learners can conclude that the rate of diffusion is affected by these factors.
3. What learning goals are associated with these diffusion simulations?
The primary learning goal is to provide an inquiry-based learning activity that allows students to experiment with and understand the concept of diffusion. Specifically, students are expected to be able to investigate and conclude how temperature and molar mass influence the rate at which diffusion occurs.
4. Who created these diffusion simulations?
These simulations were developed by the Concord Consortium, an organization known for creating open educational resources.
5. Are these simulations part of a larger collection of educational resources?
Yes, these simulations are part of the Open Educational Resources / Open Source Physics @ Singapore project, which offers a wide variety of interactive simulations and learning tools for science and mathematics education.
6. What software requirements are needed to run these diffusion simulations?
The software requirements are not explicitly mentioned in the provided text. However, given the context of other resources listed (JavaScript, HTML5 Applets), it is highly likely that these simulations are web-based and require a modern web browser that supports these technologies.
7. Are there any teacher resources or guides available for using these simulations in the classroom?
The text mentions "[SIMU_TEACHER]", which suggests that there are specific resources and guidance available for teachers to effectively integrate these diffusion simulations into their lessons. However, the actual content of these resources is not provided in the given text.
8. Where can I find other interactive simulations and open educational resources similar to these diffusion experiments?
The text highlights the "Open Educational Resources / Open Source Physics @ Singapore" project. Additionally, it mentions other platforms and creators like Easy Java/JavaScript Simulations Toolkit (Ejs), Open Source Physics by Wolfgang Christian, and various "SLS Hackathon" projects that have produced numerous interactive learning tools using JavaScript and HTML5. Exploring these mentioned names and projects would lead to a broader range of similar educational resources.