Translations
| Code |
Language |
Translator |
Run |
|
|
|
|
|
 |
Credits

 Zenia Chng; Lawrence Wee
Zenia Chng; Lawrence Wee
Source 1: Excerpts from "Balancing Chemical Equations" (Zenia Chng; Lawrence Wee, © 2022)
This source appears to be foundational material on the topic of balancing chemical equations, although the specific content of the excerpts provided is limited to the title and authorship information. However, the existence of this document suggests a theoretical or explanatory approach to the subject, likely covering the principles behind balancing equations.
Key Information:
Title: Balancing Chemical Equations
Authors: Zenia Chng; Lawrence Wee
Publication Year: 2022
Source 2: Excerpts from "Balancing Chemistry Equation JavaScript Simulation Applet HTML5 - Open Educational Resources / Open Source Physics @ Singapore"
This source describes an interactive simulation tool designed to help students learn and practice balancing chemical equations. It outlines the objectives, features, and guides for both teachers and students on how to use the applet.
Main Themes and Important Ideas:
Learning Objectives: The primary objective of the simulation is to enable students to:
"Identify in writing, the different types of of elements and number of atoms given a chemical formula and Periodic Table." This indicates an initial step of understanding chemical formulas before balancing.
"By writing the numerical coefficients, balance chemical equations, recognising that the number of atoms of the reactants must be equal to the number of atoms of the products." This explicitly states the core principle of balancing equations: the conservation of mass at the atomic level.
Simulation Features for Learning: The applet incorporates several features to aid student learning:
Multiple Levels: It offers "3 different levels of increasing difficulty" (Level 1 - 3 questions, Level 2 - 4 questions, Level 3 - 3 questions), allowing for differentiated learning and progressive skill development.
Hint Button: A "hint" button becomes active after an initial attempt, providing scaffolding by highlighting "reactant(s)/product(s) particle(s) that has/have the incorrect stoichiometric coefficient" in red. The type of red highlighting (solid or dashed) indicates whether there is an excess or insufficient number of particles.
Visual Representations: The simulation offers "3 different pictorial representations to help students visualise atoms, molecules and ion":
Default view (pictorial representation of particles).
"Show chemical symbol" view (chemical symbols and charges of ionic compounds).
"Show charges" view (representation of ions similar to 'dot-and-cross' diagrams, applicable to ionic compounds).
Immediate Feedback: A "check" button evaluates student answers. Correct answers lead to the next question, while incorrect answers require the student to try again.
Navigation: Students can toggle back and forth between attempted questions but must correctly answer the current question to proceed.
Features for Teachers: The simulation includes elements for monitoring student progress:
Log List: Two yellow boxes display a log of student answers, showing green ticks for correct answers and red crosses for incorrect ones.
Persistence of Data: The log does not reset upon clicking the "refresh" button within the simulation, but it will reset if the browser is refreshed.
Data Analytics (Simple): The log of student actions provides "simple data analytics" to help teachers give "evidence based feedback to improve students' understanding."
Question Structure: The questions progress in difficulty, with "increasing number of products on the right hand side of the equal sign." There are a total of 10 questions.
Example Questions: The source provides two example questions:
Counting Atoms in Molecules: "Question: Sulfur dioxide, SO2 can pollute the air and causes health problems. Which of the following clearly shows the number of atoms of sulfur and oxygen present in three molecules of sulfur dioxide? 3 sulfur atoms and 6 oxygen atoms" This question assesses the fundamental understanding of chemical formulas.
Balancing Combustion of Propane: "Propane ( C_{3}H_{8} ), undergoes complete combustion in excess oxygen. Which of the following is the correct balanced chemical equation for the complete combustion of propane? ( C_{3}H_{8} + 5O_{2} -> 3CO_{2} + 4H_{2}O)" This provides an example of a balanced chemical equation.
Technical Details: The simulation is a "JavaScript Simulation Applet HTML5" and is part of the "Open Educational Resources / Open Source Physics @ Singapore" project.
Accessibility and Embedding: The resource can be embedded in webpages using an iframe code provided.
Credits and License: The simulation is credited to Zenia Chng and Lawrence Wee and is licensed under the Creative Commons Attribution-Share Alike 4.0 Singapore License.
Quotes from the Source:
On the core principle: "...recognising that the number of atoms of the reactants must be equal to the number of atoms of the products."
On the hint feature: "When students click 'hint', reactant(s)/product(s) particle(s) that has/have the incorrect stoichiometric coefficient will be highlighted in red."
On feedback: "If an equation is balanced correctly, a green tick will appear on the side of the products panel. If an equation is balanced incorrectly, a red cross will appear on the side of the products panel."
On the progression of difficulty: "9 questions of different levels of difficulty (level 1, level 2 and level 3) with increasing number of products on the right hand side of the equal sign"
Potential Connections and Implications:
The combination of the "Balancing Chemical Equations" document (presumably providing the theoretical background) and the interactive simulation applet offers a potentially comprehensive learning experience for students.
The simulation's features, such as multiple levels, hints, and visual representations, cater to different learning styles and provide scaffolding for students who struggle with the concept.
The teacher monitoring features allow for formative assessment and provide insights into student understanding.
The open educational resource nature of the simulation makes it accessible for educators to integrate into their teaching materials.
Further Considerations:
The provided excerpts do not contain the actual content of the "Balancing Chemical Equations" document. Accessing this document would provide a more complete understanding of the pedagogical approach.
The effectiveness of the simulation would depend on its user-friendliness, the quality of the questions, and how well it aligns with learning objectives.
This briefing document provides an overview of the key information and themes present in the provided sources. The interactive simulation described appears to be a valuable tool for teaching and learning the fundamental skill of balancing chemical equations.
Balancing Chemical Equations: A Study Guide
Key Concepts
Chemical Equation: A symbolic representation of a chemical reaction using chemical formulas. Reactants are on the left side, and products are on the right, separated by an arrow.
Reactants: The starting materials in a chemical reaction; substances that are consumed during the reaction.
Products: The substances that are formed as a result of a chemical reaction.
Law of Conservation of Mass: Matter cannot be created or destroyed in a chemical reaction. This means the number of atoms of each element must be the same on both sides of a balanced chemical equation.
Balanced Chemical Equation: A chemical equation in which the number of atoms for each element in the reaction is the same in both the reactants and the products. This is achieved by adding numerical coefficients in front of the chemical formulas.
Chemical Formula: A representation of a molecule or ionic compound that shows the types of atoms and their ratios using chemical symbols and subscripts.
Atom: The smallest unit of an element that retains the chemical properties of that element.
Molecule: A group of two or more atoms held together by chemical bonds. These atoms can be of the same element (e.g., O₂) or different elements (e.g., H₂O).
Ion: An atom or molecule that has gained or lost one or more electrons, giving it an electrical charge (positive or negative).
Coefficient: A whole number placed in front of a chemical formula in a chemical equation to indicate the number of moles (or molecules) of that substance involved in the reaction. Coefficients are used to balance chemical equations.
Subscript: A number written below and to the right of a chemical symbol in a chemical formula. It indicates the number of atoms of that element in one unit of the substance. Subscripts cannot be changed when balancing equations.
Stoichiometric Coefficient: The numerical coefficient in a balanced chemical equation that indicates the relative amounts of reactants and products in terms of moles.
Study Questions
What is the purpose of a chemical equation? What information does it convey about a chemical reaction?
Explain the law of conservation of mass and why it is important in balancing chemical equations.
What is the difference between reactants and products in a chemical reaction? How are they represented in a chemical equation?
Define a balanced chemical equation. Why is it necessary to have balanced chemical equations?
What is the role of coefficients in balancing chemical equations? Can subscripts in chemical formulas be changed during the balancing process? Explain your reasoning.
Describe the steps involved in balancing a chemical equation. Provide an example of an unbalanced equation and show the process of balancing it.
How can you determine the number of atoms of each element present in a given chemical formula with a coefficient? For example, how many sulfur and oxygen atoms are present in 3SO₂?
What is a chemical formula, and what information does the subscript within a chemical formula provide?
How can a simulation tool aid in understanding and practicing the balancing of chemical equations? What features might such a simulation include to help students learn?
What are some common mistakes to avoid when balancing chemical equations?
Quiz
Explain the fundamental principle that necessitates the balancing of chemical equations. Why can't we simply have unequal numbers of atoms on both sides of the reaction?
Consider the unbalanced equation: H₂ + O₂ → H₂O. Identify the reactants and products. Explain why this equation is unbalanced in terms of the number of hydrogen and oxygen atoms.
What is the role of coefficients in the process of balancing a chemical equation? Provide a brief example of how adding a coefficient changes the number of atoms of an element.
A chemical formula for glucose is C₆H₁₂O₆. If you have two molecules of glucose, how many atoms of carbon, hydrogen, and oxygen are present in total? Show your calculation.
Describe one feature of the "Balancing Chemistry Equation JavaScript Simulation Applet HTML5" that you think would be most helpful for students learning to balance equations and explain why.
What is the difference between a subscript and a coefficient in a chemical equation? Which one can be changed when balancing an equation, and why?
What does it mean when a chemical equation has a "green tick" next to the products panel in the simulation described in the source material? What does a "red cross" indicate?
Explain the purpose of the "hint" button in the simulation. How does it visually indicate an imbalance in the number of reactant or product particles?
The balanced equation for the combustion of methane is CH₄ + 2O₂ → CO₂ + 2H₂O. Identify the stoichiometric coefficients for each reactant and product in this reaction.
Why is it important for teachers to have access to a log of students' responses when using a simulation for balancing chemical equations? What kind of information can this log provide?
Quiz Answer Key
The fundamental principle that necessitates balancing chemical equations is the Law of Conservation of Mass, which states that matter cannot be created or destroyed in a chemical reaction. Therefore, the number of atoms of each element must be equal on both the reactant and product sides to reflect that the same atoms are present before and after the reaction, just rearranged.
In the unbalanced equation H₂ + O₂ → H₂O, the reactants are hydrogen (H₂) and oxygen (O₂), and the product is water (H₂O). This equation is unbalanced because there are 2 oxygen atoms on the reactant side but only 1 oxygen atom on the product side. While there are 2 hydrogen atoms on both sides, the oxygen atoms are not conserved.
Coefficients are whole numbers placed in front of chemical formulas in an equation to multiply the number of atoms of each element in that formula. For example, in 2H₂O, the coefficient '2' means there are 2 * 2 = 4 hydrogen atoms and 2 * 1 = 2 oxygen atoms.
In one molecule of glucose (C₆H₁₂O₆), there are 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. Therefore, in two molecules of glucose, there would be 2 * 6 = 12 carbon atoms, 2 * 12 = 24 hydrogen atoms, and 2 * 6 = 12 oxygen atoms.
One helpful feature of the simulation is the visual representation of particles. This allows students to see the actual atoms and molecules involved in the reaction, making the abstract concept of balancing equations more concrete and understandable, especially when the "hint" button highlights imbalances visually.
A subscript is a number within a chemical formula that indicates the number of atoms of a particular element in one unit of that substance. A coefficient is a number placed in front of the entire chemical formula, indicating the number of molecules or moles of that substance. Only coefficients can be changed when balancing an equation because changing subscripts would alter the chemical identity of the substances involved.
A "green tick" next to the products panel in the simulation indicates that the student has correctly balanced the chemical equation presented. Conversely, a "red cross" indicates that the equation as entered by the student is not balanced.
The "hint" button in the simulation helps students by highlighting reactant(s) or product(s) particles with incorrect stoichiometric coefficients in red. Solid red lines indicate an excess number of particles, while dashed red lines indicate an insufficient number of particles, guiding students on how to adjust the coefficients.
In the balanced equation CH₄ + 2O₂ → CO₂ + 2H₂O, the stoichiometric coefficients are: 1 for methane (CH₄), 2 for oxygen (O₂), 1 for carbon dioxide (CO₂), and 2 for water (H₂O). Note that when no coefficient is written, it is understood to be 1.
A log of students' responses can provide teachers with valuable data on student progress, common errors, and areas of difficulty. This allows teachers to give evidence-based feedback and tailor their instruction to improve students' understanding of balancing chemical equations.
Essay Format Questions
Discuss the significance of the Law of Conservation of Mass in the context of chemical reactions and explain how the process of balancing chemical equations directly reflects this fundamental law. Use examples to illustrate your points.
Describe the key features of a digital simulation tool, such as the one mentioned in the source material, and analyze how these features can effectively support student learning and understanding of balancing chemical equations.
Explain the difference between subscripts and coefficients in chemical equations and discuss why only coefficients can be manipulated during the balancing process. What would be the implications of changing subscripts?
Outline a step-by-step strategy for balancing chemical equations, using a specific unbalanced equation as an example to demonstrate each step. What are some common pitfalls students encounter, and how can they be avoided?
Considering the objectives outlined in the source material, analyze how the ability to balance chemical equations contributes to a broader understanding of chemistry and chemical reactions. Why is this a fundamental skill in the study of chemistry?
Glossary of Key Terms
Atom: The basic building block of matter, consisting of a nucleus (containing protons and neutrons) surrounded by electrons. It is the smallest unit of an element that can participate in a chemical reaction.
Balanced Chemical Equation: A chemical equation where the number of atoms of each element is equal on both the reactant and product sides, satisfying the Law of Conservation of Mass.
Chemical Equation: A symbolic representation of a chemical reaction using chemical formulas, with reactants on the left and products on the right, connected by an arrow.
Chemical Formula: A notation that uses chemical symbols and subscripts to indicate the number and types of atoms in a molecule or the simplest repeating unit of an ionic compound.
Coefficient: A whole number placed in front of a chemical formula in a chemical equation to indicate the relative number of moles (or molecules) of that substance participating in the reaction. Used to balance equations.
Ion: An atom or molecule that has acquired an electrical charge by gaining or losing one or more electrons. Positively charged ions are called cations, and negatively charged ions are called anions.
Law of Conservation of Mass: A fundamental principle in chemistry stating that matter cannot be created or destroyed in a chemical reaction. The total mass of the reactants must equal the total mass of the products.
Molecule: A group of two or more atoms held together by chemical bonds. It is the smallest unit of a covalent compound that retains the characteristic properties of that compound.
Product: A substance that is formed as a result of a chemical reaction. Products are written on the right side of a chemical equation.
Reactant: A substance that is consumed during a chemical reaction. Reactants are written on the left side of a chemical equation.
Stoichiometric Coefficient: The numerical coefficient in a balanced chemical equation that represents the molar ratio of reactants and products.
Subscript: A number written below and to the right of a chemical symbol in a chemical formula, indicating the number of atoms of that element present in one unit of the substance. Subscripts define the chemical identity and should not be changed when balancing equations.
New Data Analytics
Launch URL https://iwant2study.org/moodle402/enrol/lti/launch.php
Custom properties id=6ddc0320-d752-46b7-b771-10b3b8808024
Together https://iwant2study.org/moodle402/enrol/lti/launch.php?id=6ddc0320-d752-46b7-b771-10b3b8808024
Objectives
You should now be able to:
- Identify in writing, the different types of of elements and number of atoms given a chemical formula and Periodic Table.
- By writing the numerical coefficients, balance chemical equations, recognising that the number of atoms of the reactants must be equal to the number of atoms of the products.
Brief Description
It is a Chemistry simulation on balancing chemical equations - 10 questions, 3 different levels, with a log list for teachers to track student's responses and a "hint" button and pop-up boxes to scaffold the learning of students. In addition, there are 3 different pictorial representations to help students visualise atoms, molecules and ion
Guide for teachers using the simulation
- There are 3 different levels of increasing difficulty. (Level 1 - 3 questions, Level 2 - 4 questions, Level 3 - 3 questions).
- The "hint" button is only enabled after students have made an attempt on the question (i.e. after clicking on "check" once). Upon answering a question correct, the next question will be shown. Students are able to toggle back and forth the questions they have attempted, but will not be able to proceed if they have not cleared the current question.
- When students click "hint", reactant(s)/product(s) particle(s) that has/have the incorrect stoichiometric coefficient will be highlighted in red.
- particles highlighted in solid red lines - excess number of particles
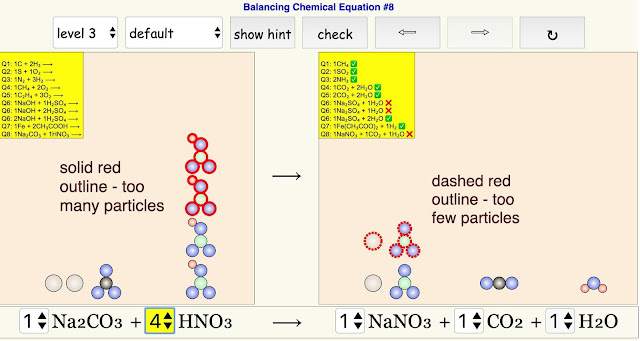
- particles highlighted in dashed red lines - insufficient number of particles
- There are 3 different views available for students - default, show chemical symbol and show charges
- The default view shows the pictorial representation of particles, available for all 3 levels.
- "Show chemical symbol" is also available for all 3 levels, but it shows the chemical symbol of each particle (including charges of ionic compounds).
- "Show charges" is only applicable to questions with ionic compounds (i.e. question 6 onwards). Its representation of ions is similar to that of 'dot-and-cross' diagrams.
- For monitoring of student's progress, there are two yellow boxes found on the top left hand corner of the reactant and product boxes. It shows the log of student's answers.
- If an equation is balanced correctly, a green tick will appear on the side of the products panel.
- If an equation is balanced incorrectly, a red cross will appear on the side of the products panel.
- If a student click on the "refresh" button in the simulation, the log will not reset. However, if the student was to refresh the browser, the log will automatically be refreshed as well.
Additional notes
-
9 questions of different levels of difficulty (level 1, level 2 and level 3) with increasing number of products on the right hand side of the equal sign
- show hint button, temporary reveals the shortage (dash outlines) or extra (solid outline) atoms
- check button, evaluate the students' choice on the bottom combobox numbers, when correct, the next question will be fielded
- left arrow button, to allow students to revisit questions they got correct
- right arrow button, to allow students to progress only after they got the questions correct, but not before
- visualisation panels of up to 6 molecules on the left (reactants) and right (products) of the reaction
- simple data analytics of the students' actions, that stays on screen even after reset button, to help teachers' give evidence based feedback to improve students' understanding.
Guide for students
In the simulation, there are 10 questions of increasing difficulty. After you have balanced the chemical equation, you will be shown the next question. You will only be allowed to toggle between levels and questions after you have answered them correctly for the first time.
The “hint” button will only be active after you have attempted the question once. To reveal the hint, click and hold on the button.
If you see a solid red outline around the particle, it would mean there are too many particles for that reactant/product. A dashed red outline refers to too few particles.
There are three different pictorial representation for you to choose from – default view, particles with chemical symbols, and particles represented as ions.
Question: Sulfur dioxide, SO2 can pollute the air and causes health problems. Which of the following clearly shows the number of atoms of sulfur and oxygen present in three molecules of sulfur dioxide?
3 sulfur atoms and 6 oxygen atoms
Propane \( C_{3}H_{8} \), undergoes complete combustion in excess oxygen. Which of the following is the correct balanced chemical equation for the complete combustion of propane?
\( C_{3}H_{8} + 5O_{2} -> 3CO_{2} + 4H_{2}O\)
Video
[text]
Version:
- https://weelookang.blogspot.com/2019/11/balancing-chemistry-equation-javascript.html
- https://vle.learning.moe.edu.sg/community-gallery/lesson/view/c2cd909f-5ed7-49f3-9959-3ac4bcf6a71f SLS lesson
Other Resources
[text]
Frequently Asked Questions: Balancing Chemical Equations
1. What is the fundamental principle behind balancing chemical equations?
Balancing chemical equations is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. This means that the number of atoms of each element must be the same on both the reactant (starting materials) and product (substances formed) sides of the chemical equation.
2. Why is it important to balance chemical equations?
Balancing chemical equations is crucial for accurately representing chemical reactions. A balanced equation provides the correct stoichiometric ratios between reactants and products, which are essential for calculating amounts of substances needed or produced in a reaction. Without a balanced equation, predictions about the quantities involved in a chemical reaction would be incorrect, making it impossible to perform accurate chemical calculations or understand the true nature of the reaction.
3. How do you balance a chemical equation?
Balancing a chemical equation involves adjusting the numerical coefficients placed in front of the chemical formulas of reactants and products. The process typically involves these steps: * Identify all the elements present in the reaction. * Count the number of atoms of each element on both the reactant and product sides. * Begin by balancing one element at a time by placing appropriate coefficients in front of the chemical formulas containing that element. It's often helpful to start with elements that appear in only one reactant and one product. * Continue balancing other elements, adjusting coefficients as needed. Be aware that changing the coefficient of one compound can affect the balance of other elements. * Once all elements appear to be balanced, double-check the number of atoms of each element on both sides of the equation to ensure they are equal. * The coefficients should be the smallest whole numbers possible. If you end up with fractional coefficients, you can multiply the entire equation by the smallest common multiple to obtain whole numbers.
4. What is the role of coefficients in a balanced chemical equation?
Coefficients in a balanced chemical equation represent the relative number of moles (or molecules) of each reactant and product involved in the reaction. For example, in the balanced equation (2H_2 + O_2 \rightarrow 2H_2O), the coefficients indicate that two molecules of hydrogen react with one molecule of oxygen to produce two molecules of water. These coefficients establish the stoichiometry of the reaction, providing the quantitative relationships between the reacting substances.
5. What are subscripts in a chemical formula, and can they be changed when balancing equations?
Subscripts within a chemical formula indicate the fixed ratio of atoms within a molecule or formula unit of a specific substance. For instance, in water ((H_2O)), the subscript '2' indicates that each molecule of water contains two hydrogen atoms and one oxygen atom. When balancing chemical equations, subscripts must never be changed, as doing so would alter the identity of the substance. Only the coefficients in front of the chemical formulas can be adjusted to balance the number of atoms of each element.
6. What are some common challenges or difficulties students face when learning to balance chemical equations?
Students often face several challenges when learning to balance chemical equations, including: * Miscounting the number of atoms of each element, especially when polyatomic ions or complex formulas are involved. * Confusing subscripts (which cannot be changed) with coefficients (which are adjusted for balancing). * Using trial-and-error without a systematic approach, which can become inefficient for more complex equations. * Not understanding the underlying principle of conservation of mass. * Difficulties in handling equations with multiple elements that require iterative adjustments of coefficients.
7. How can interactive simulations and visual representations aid in understanding and mastering balancing chemical equations?
Interactive simulations and visual representations can be highly effective tools for learning to balance chemical equations. They can: * Provide a visual representation of atoms and molecules, making the concept of conservation of mass more concrete. * Allow students to manipulate coefficients and immediately see the impact on the number of atoms on each side of the equation. * Offer hints and feedback, such as highlighting elements that are not balanced or indicating whether there are too many or too few particles of a particular reactant or product. * Enable students to work through problems at their own pace and revisit questions as needed. * Cater to different learning styles by offering various representations, such as pictorial views, chemical symbols, and representations of ionic charges. * Provide a structured learning experience with increasing levels of difficulty, allowing students to build their skills progressively.
8. Besides balancing, what other fundamental concepts in chemistry are closely related to chemical equations?
Balancing chemical equations is a foundational skill that connects to several other key concepts in chemistry, including: * Stoichiometry: The quantitative relationships between reactants and products in chemical reactions, which relies directly on the balanced equation and its coefficients for calculations involving moles, mass, and volume. * Types of Chemical Reactions: Recognizing different patterns of reactions (e.g., synthesis, decomposition, single replacement, double replacement, combustion) often precedes the balancing process. * Chemical Formulas and Nomenclature: Understanding how to write correct chemical formulas and name compounds is essential for interpreting and writing chemical equations that need to be balanced. * The Mole Concept: The coefficients in a balanced equation represent molar ratios, making the mole concept crucial for applying balanced equations to real-world quantities. * Limiting Reactants and Percent Yield: These concepts build upon stoichiometric calculations derived from balanced chemical equations to determine the maximum amount of product that can be formed and the efficiency of a reaction.


Zenia Chng; Lawrence Wee



.png
)





