About
Brownian motion
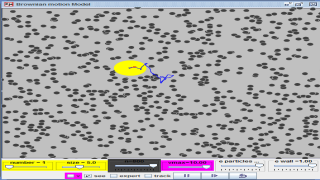
This is a simulation of brownian motion of a particle that collides with a large set of smaller particles which move with uniform motion in different random directions.
For more info:
http://weelookang.blogspot.sg/2010/06/ejs-open-source-brownian-motion-gas.html
Original Author:
Simulación preparada por Francisco Esquembre para el libro
Creación de Simulaciones Interactivas en Java.
Aplicación
a la Enseñanza de la Física
(C) Pearson Educación 2004.
Modified by Fu-Kwun Hwang
http://www.phy.ntnu.edu.tw/ntnujava/
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
| ru | ru | ru |  |
|
Credits


 Francisco Esquembre; Fu-Kwun Hwang; lookang
Francisco Esquembre; Fu-Kwun Hwang; lookang
Briefing Document: Brownian Motion & Diffusion Educational Resources
1. Overview
This document summarizes information from the "Open Educational Resources / Open Source Physics @ Singapore" website, specifically focusing on their resources related to Brownian motion, diffusion, and related physics concepts. The website offers a wealth of interactive simulations and learning tools, primarily built using Easy JavaScript Simulation (EJS) and Molecular Workbench (MW) platforms, aimed at enhancing the understanding of complex physical phenomena.
2. Key Themes and Concepts
- Brownian Motion:
- Definition: The random movement of particles suspended in a fluid (liquid or gas) caused by collisions with fast-moving atoms or molecules of the fluid. The document defines it as "the random motion of particles suspended in a fluid (a liquid or a gas) resulting from their collision with the fast-moving atoms or molecules in the gas or liquid."
- Historical Context: The phenomenon is named after Robert Brown, who observed it in pollen grains. The document highlights that "in 1827, while looking through a microscope at particles trapped in cavities inside pollen grains in water, he noted that the particles moved through the water."
- Einstein's Explanation: Albert Einstein explained Brownian motion as being caused by the movement of individual water molecules. The document specifically states, "Albert Einstein published a paper in 1905 that explained in precise detail how the motion that Brown had observed was a result of the pollen being moved by individual water molecules." This explanation provided crucial evidence for the existence of atoms and molecules.
- Random Nature: The direction of the force exerted by the colliding molecules is constantly changing, resulting in the erratic, "random" movement of the suspended particle.
- Stochastic Process: Brownian motion is described as a "continuous-time stochastic (or probabilistic) processes," highlighting its fundamental nature in statistical physics.
- Diffusion:
- Description: The movement of molecules from an area of high concentration to an area of low concentration. The document illustrates diffusion by saying "Add a drop of dye anywhere in the container, and watch it diffuse through the water."
- Temperature's Influence: The rate of diffusion increases with temperature. The interactive model is designed to "Explore the role of temperature on the rate of diffusion."
- Molecular Mass's Influence: The rate of diffusion decreases as the mass of the diffusing molecules increases. The interactive model allows the user to "Explore the role of molecular mass on the rate of diffusion."
- Permeability: Pore size of membranes affects which molecules can pass through. This aspect is explored in the interactive "How does pore size affect the diffusion of different molecules?"
- Biological Significance: The document explains how diffusion is critical for gas exchange (oxygen and carbon dioxide) in cell membranes, pointing out that "Oxygen and carbon dioxide are two molecules that can freely cross the cell membrane. In aerobic cells, oxygen is necessary for cell functioning and carbon dioxide is produced as a waste molecule."
- Interactive Simulations and Models:
- EJS (Easy JavaScript Simulation): A core component of the website's interactive content, EJS is used to create dynamic models of various physical phenomena, including Brownian motion. The document mentions "EasyJavaScriptSimulation" as a resource.
- Molecular Workbench (MW): Another platform for creating interactive simulations, often used for exploring molecular-level interactions. The document mentions "MolecularWorkbench" as a resource.
- Accessibility: Simulations are designed to be accessible on various platforms including "Android/iOS including handphones/Tablets/iPads" and "Windows/MacOSX/Linux including Laptops/Desktops" as well as "ChromeBook Laptops"
- Specific Simulations: The document provides links to various interactive models such as the Brownian motion simulation itself, diffusion models examining temperature, mass, and membrane permeability, among many other physics topics. This is important for a practical understand of the abstract concepts.
3. Key Ideas and Facts
- Direct Observation & Modeling: The resources promote active learning through the use of interactive simulations. Learners can manipulate variables such as temperature, particle mass, and pore size and immediately observe the effects on the simulated system.
- Evidence for Atomic Theory: The document emphasizes that the observation and explanation of Brownian Motion served as direct evidence for the existence of atoms and molecules.
- Interconnectedness of Concepts: The resource demonstrates the interrelation of concepts, for example, linking Brownian motion (the movement of a particle) with diffusion (the movement of a concentration) and how they both stem from the kinetic theory of matter.
- Stochasticity in Nature: Brownian motion highlights the inherent randomness in many physical processes at the molecular level.
- Importance of Diffusion: The resources illustrate how fundamental the process of diffusion is in both physical and biological systems.
4. Quotes from Source Material:
- "Brownian motion or pedesis... is the random motion of particles suspended in a fluid (a liquid or a gas) resulting from their collision with the fast-moving atoms or molecules in the gas or liquid." (Definition of Brownian motion)
- "In 1827, while looking through a microscope at particles trapped in cavities inside pollen grains in water, he noted that the particles moved through the water." (Description of Robert Brown's observations)
- "Albert Einstein published a paper in 1905 that explained in precise detail how the motion that Brown had observed was a result of the pollen being moved by individual water molecules." (Einstein's explanation of Brownian motion)
- "Add a drop of dye anywhere in the container, and watch it diffuse through the water." (Description of Diffusion)
5. Additional Points
- Open Educational Resources: The website is dedicated to providing free and open educational resources, allowing access to these interactive simulations for anyone.
- Versatility: The simulations are designed for secondary school students, making complex physics concepts accessible.
- Extensive Resources: The site boasts numerous other simulations across physics, mathematics and other subjects.
6. Conclusion
The "Open Educational Resources / Open Source Physics @ Singapore" website provides valuable educational tools for exploring complex physical phenomena like Brownian motion and diffusion. The interactive simulations, coupled with historical and theoretical background, offer an engaging and effective approach for learning these concepts. The site emphasizes hands-on experimentation through simulation, solidifying the learners understanding of statistical mechanics, diffusion and gas dynamics.
Brownian Motion Study Guide
Quiz
Instructions: Answer the following questions in 2-3 sentences each.
- What is Brownian motion, and what causes it?
- Who was Robert Brown, and what did he observe that led to the discovery of Brownian motion?
- How did Albert Einstein contribute to the understanding of Brownian motion?
- What is the significance of Jean Perrin's work in relation to Brownian motion?
- According to the simulation, what is the relationship between the size of particles and the way they move in Brownian motion?
- How does the simulation model the random collisions that cause Brownian motion?
- What does the text mean by saying that Brownian motion is a "continuous-time stochastic process?"
- According to the source, how does temperature affect the rate of diffusion?
- How does molecular mass influence the rate of diffusion, as illustrated in the simulations?
- How can a biological membrane's pore size affect the diffusion of different molecules?
Quiz Answer Key
- Brownian motion is the random movement of particles suspended in a fluid, like a liquid or a gas. It is caused by the collisions of these particles with the fast-moving atoms or molecules of the surrounding fluid.
- Robert Brown was a botanist who, in 1827, observed the random movement of particles trapped inside pollen grains in water under a microscope. His observations were the first documentation of what became known as Brownian motion.
- Albert Einstein published a paper in 1905 that explained how the motion observed by Brown was a result of the pollen being bombarded by individual water molecules. His work provided a theoretical basis for the phenomenon and provided evidence for the existence of molecules.
- Jean Perrin experimentally verified Einstein's theoretical explanation of Brownian motion in 1908. His work provided definitive empirical evidence of atoms and molecules existence, which earned him the Nobel Prize in Physics in 1926.
- The simulation models Brownian motion as the motion of a large particle being impacted by a swarm of smaller particles. The large particle's random movement is due to it being struck by the smaller particles, causing an irregular motion pattern.
- The simulation models random collisions by using a large number of small particles moving randomly and impacting a larger particle. The force of these impacts changes continuously, leading to the larger particle's seemingly random motion.
- The term “continuous-time stochastic process” means that Brownian motion is a random process that occurs over a period of time, with no clear pattern, and is a key idea in physics,
- According to the source, temperature increases the rate of diffusion. In the simulation, molecules diffuse at a faster rate as the temperature rises.
- The simulation suggests that molecular mass has an inverse relationship to diffusion rate. Lighter molecules diffuse more rapidly, while heavier molecules diffuse at a slower pace.
- A biological membrane's pore size determines which molecules can cross. Larger molecules are unable to pass through smaller pores, while smaller molecules can cross more easily.
Essay Questions
Instructions: Answer the following questions in essay format, using your understanding of the source material.
- Explain the historical context surrounding the discovery and understanding of Brownian motion. Discuss the contributions of Robert Brown, Albert Einstein, and Jean Perrin, and explain why their work was important for understanding the nature of matter.
- Using the simulations and information provided in the source, describe how Brownian motion can be considered a model for diffusion. Discuss the factors that influence the rate of diffusion, such as temperature and molecular mass.
- Analyze the various applications of Brownian motion. How does understanding this phenomenon help us to study and understand the behavior of matter?
- Discuss how the simulations and resources provided enhance the learning experience of Brownian motion compared to traditional instruction. In what ways do these interactive models and tools support conceptual understanding of physical science?
- Biological membranes, as described in the source, are selectively permeable. How does understanding Brownian motion help us explain the movement of molecules across these membranes? Give specific examples from the source, and relate them to concepts of diffusion.
Glossary
- Brownian Motion: The random movement of particles suspended in a fluid (a liquid or a gas) caused by their collision with the fast-moving atoms or molecules in the fluid.
- Diffusion: The net movement of particles from an area of higher concentration to an area of lower concentration. It is a process that results from the random movement of molecules.
- Kinetic Model: A model that explains the behavior of matter in terms of the motion of its particles. For example, the kinetic model of gases states that gas particles are in constant random motion.
- Pedeses: An alternative term for Brownian motion, derived from the ancient Greek word meaning "leaping."
- Stochastic Process: A random process where the system's future state is not fully predictable. In physics, these processes use the language of probabilities and randomness.
- Molecular Mass: The mass of a molecule, calculated by adding up the masses of all the atoms that make up the molecule.
- Permeable Membrane: A membrane that allows molecules to pass through it.
- Selectively Permeable Membrane: A biological membrane that permits some molecules to pass while restricting the passage of others.
- Cell Membrane: The membrane surrounding cells; it is composed of a phospholipid bilayer that is selectively permeable.
- Phospholipid Bilayer: The structure of cell membranes, formed by two layers of phospholipid molecules, that allows some molecules to pass through but not others.
Apps

https://play.google.com/store/apps/details?id=com.ionicframework.brownianapp183853
Introduction
Brownian motion or pedesis (from Ancient Greek: πήδησις/pέːdεːsis/ "leaping") is the random motion of particles suspended in a fluid (a liquid or a gas) resulting from their collision with the fast-moving atoms or molecules in the gas or liquid.[1] This transport phenomenon is named after the botanist Robert Brown. In 1827, while looking through a microscope at particles trapped in cavities inside pollen grains in water, he noted that the particles moved through the water. Atoms and molecules had long been theorized as the constituents of matter, and Albert Einstein published a paper in 1905 that explained in precise detail how the motion that Brown had observed was a result of the pollen being moved by individual water molecules. This explanation of Brownian motion served as convincing evidence that atoms and molecules exist, and was further verified experimentally by Jean Perrin in 1908. Perrin was awarded the Nobel Prize in Physics in 1926 "for his work on the discontinuous structure of matter" (Einstein had received the award five years earlier "for his services to theoretical physics" with specific citation of different research). The direction of the force of atomic bombardment is constantly changing, and at different times the particle is hit more on one side than another, leading to the seemingly random nature of the motion.
Brownian motion is among the simplest of the continuous-time stochastic (or probabilistic) processes, a big idea in physics.
Other Resources
http://mw.concord.org/nextgen/interactives/
surprisingly similiar random movement between brownian and diffusion.
Add a drop of dye anywhere in the container, and watch it diffuse through the water.
Click in the model to add a drop of dye. Watch how the molecules move through the water. Trace an individual molecule to see how it moves through the liquid.
http://lab.concord.org/embeddable.html#interactives/sam/diffusion/1-dropping-dye-on-click.json
How does temperature affect the rate of diffusion?
Explore the role of temperature on the rate of diffusion. Set the temperature, then remove the barrier, and measure the amount of time it takes the blue molecules to reach the gas sensor. When the gas sensor has detected three blue molecules, it will stop the experiment. Compare the diffusion rates at low, medium and high temperatures. Trace an individual molecule to see the path it takes.
http://lab.concord.org/embeddable.html#interactives/sam/diffusion/2-temperature.json
How does molecular mass affect the rate of diffusion?
Explore the role of molecular mass on the rate of diffusion. Select the mass of the molecules behind the barrier. Remove the barrier, and measure the amount of time it takes the molecules to reach the gas sensor. When the gas sensor has detected three molecules, it will stop the experiment. Compare the diffusion rates of the lightest, heavier and heaviest molecules. Trace an individual molecule to see the path it takes.
http://lab.concord.org/embeddable.html#interactives/sam/diffusion/3-mass.json
How does pore size affect the diffusion of different molecules?
Biological membranes are selectively permeable; some molecules can cross while others cannot. One way to affect this is through pore size. Change the pore size with the slider to change the permeability of the membrane to the different types of molecules. Trace an individual molecule to see the path it takes.
Cell membranes are composed of two layers of phospholipids (a phospholipid bilayer). Some molecules are capable of crossing this membrane directly, without use of specific membrane channels.
Oxygen and carbon dioxide are two molecules that can freely cross the cell membrane. In aerobic cells, oxygen is necessary for cell functioning and carbon dioxide is produced as a waste molecule. Hence, the cell “wants” oxygen to enter and carbon dioxide to leave. But molecules don’t move only in one direction–they diffuse randomly across the membrane.
Set up the model with high oxygen and low carbon dioxide outside the cell and low oxygen and high carbon dioxide inside the cell. In which direction do the oxygen and carbon dioxide molecules move?
http://lab.concord.org/embeddable.html#interactives/sam/diffusion/5-permeable-membrane.json
How does pore size affect the diffusion of different molecules?
Biological membranes are selectively permeable; some molecules can cross while others cannot. One way to affect this is through pore size. Change the pore size with the slider to change the permeability of the membranes to the different types of molecules. Trace an individual molecule to see the path it takes.
Video
https://www.youtube.com/watch?v=gPMVaAnij88 by STEM Learning
Versions:
- https://weelookang.blogspot.com/2020/06/brownian-motion-gas-model-secondary.html
- https://weelookang.blogspot.com/2015/08/ejss-brownian-motion-model.html
- https://weelookang.blogspot.com/2015/10/thank-you-for-usa-china-phd-student.html
- https://weelookang.blogspot.com/2010/06/ejs-open-source-brownian-motion-gas.html
Other resources
- http://www.phy.ntnu.edu.tw/ntnujava/index.php?topic=1121.0 simplied flu spreading model by Fu-Kwun Hwang
- https://deck.toys/decks/71hIDEePF/Brownian-Motion-Differentiation-of-Product by Amos
Frequently Asked Questions About Brownian Motion and Diffusion
- What is Brownian motion and how was it discovered? Brownian motion is the random movement of particles suspended in a fluid (liquid or gas). It arises from the collisions of these particles with the fast-moving atoms or molecules of the surrounding fluid. It was first noted by botanist Robert Brown in 1827 while observing pollen grains in water under a microscope. However, it was Albert Einstein in 1905 who provided the theoretical explanation, demonstrating how this motion is a consequence of the constant bombardment by individual molecules. This explanation was later experimentally confirmed by Jean Perrin.
- How does the simulation model Brownian motion? The simulation uses a model where a larger particle collides with many smaller particles that move in random directions at uniform speeds. This interaction mimics the bombardment of a larger particle by molecules in a fluid, causing its seemingly erratic path. This provides a visual and interactive way to understand how the collisions drive Brownian motion.
- What is the significance of Brownian motion in scientific understanding? Brownian motion is crucial because its explanation provided strong evidence for the existence of atoms and molecules, at a time when they were still theoretical constructs for some. Einstein’s mathematical description of Brownian motion was a key contribution, and this understanding earned Perrin the Nobel Prize in Physics. Furthermore, it is a simple example of a continuous-time stochastic process that is important in physics.
- How is diffusion related to Brownian motion? Diffusion and Brownian motion are closely related. Diffusion describes the net movement of molecules from an area of higher concentration to an area of lower concentration. Brownian motion is the random movement of individual particles that underlies diffusion. In the context of these simulations, by dropping a drop of dye into water, we can see how both Brownian motion of individual particles and the overall effect of diffusion cause the dye to spread out in the water over time. Both concepts rely on the random motion of molecules.
- How does temperature affect the rate of diffusion? Temperature plays a significant role in the rate of diffusion. The simulations show that at higher temperatures, the molecules move faster, and therefore, the process of diffusion is sped up. Higher temperature implies more kinetic energy. This results in more frequent and energetic collisions and a faster net movement of molecules.
- How does molecular mass affect the rate of diffusion? Molecular mass is inversely related to the rate of diffusion. Lighter molecules diffuse faster because they have greater average speeds at the same temperature. Simulations show that lighter molecules will reach a sensor faster than heavier molecules, due to the greater ease of movement they have within the medium.
- What is the role of pore size in diffusion, especially in cell membranes? Pore size is critical for regulating the diffusion of molecules across biological membranes, which are selectively permeable. Some molecules can cross the membrane directly, while others require specific channels. By varying the pore size in the simulation, one can observe how the membrane’s permeability affects the movement of different-sized molecules. This allows for some molecules to pass through and some to be blocked, and this selective process is essential for cell functioning.
- How does the simulation model gas exchange across a cell membrane? The simulation models gas exchange by showcasing the movement of oxygen and carbon dioxide molecules across a cell membrane, driven by concentration differences. If you start with high oxygen outside the cell and low inside the cell, oxygen will randomly diffuse into the cell and vice versa for carbon dioxide. This demonstrates how gases can move across cell membranes without specific channels or active transport, driven by the random motion of diffusion.
- Details
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 11006