About

Molecular Dynamics JS Performance Model
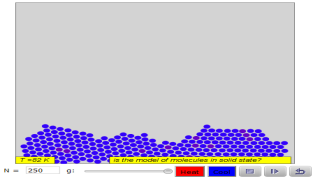
The Molecular Dynamics JavaScript Performance Model computes the trajectory of particles acted on by a Lennard-Jones force. This simulation is designed to test the speed of JavaScript for a computationally intensive model. The user can vary the number of particles, the number of frames per second, and the number of Verlet steps between frames. The actual number of Verlet steps per frame is shown.
Note: If the model becomes unstable, reset the model and reduce the computational timestep dt.
Credits:
The Molecular Dynamics JavaScript Performance Model was developed by Wolfgang Christian and Francisco Esquembre using version 5 of the Easy Java Simulations (EJS 5) modeling tool. Although EJS is a Java program, EJS 5 creates stand alone JavaScript programs that run in almost any PC or tablet browser. Information about EJS is available at: <http://www.um.es/fem/Ejs/> and in the OSP ComPADRE collection <http://www.compadre.org/OSP/>.
http://weelookang.blogspot.sg/2014/10/ejss-three-state-of-matter-model.html
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits


 Wolfgang Christian; Francisco Esquembre; lookang
Wolfgang Christian; Francisco Esquembre; lookang
Briefing Document: ⚛️Open Educational Resources / Open Source Physics @ Singapore - Interactive Simulations
Date: October 25, 2024
Subject: Analysis of Interactive Physics and Math Simulations Platform
Source: Excerpts from "Super Powerful Simulator for Two Atoms..." on the Open Educational Resources / Open Source Physics @ Singapore website
Overview:
This document summarizes the key features, themes, and educational resources found on the Open Educational Resources / Open Source Physics @ Singapore website, specifically focusing on the "Super Powerful Simulator" and related interactive simulations. The platform offers a vast collection of simulations built with Easy JavaScript Simulations (EJS), targeting a wide range of educational levels from primary school to junior college. The primary focus is on providing interactive, dynamic visualizations to aid in understanding complex physics and math concepts.
Key Themes and Ideas:
- Interactive Learning: The platform emphasizes hands-on, interactive learning through simulations. Instead of passive reading, students can directly manipulate parameters and observe the outcomes. The resources encourage active engagement with the material.
- Example: The "Super Powerful Simulator" allows users to vary the number of particles, frames per second, and Verlet steps, observing the impact on molecular dynamics.
- Wide Range of Topics: The simulations cover a vast range of topics in physics and mathematics, including:
- Molecular Dynamics: Simulations exploring the behavior of atoms and molecules in different states of matter (gas, liquid, solid) and under various forces (Lennard-Jones force).
- Quote: "Explore how molecules in a gas move relative to each other. 'Mark two atoms,' then run the model to see how the molecules move relative to each other."
- Mechanics: Including simple harmonic motion, projectile motion, gravitational fields, collisions, and frictional forces.
- Waves: Superposition of waves, diffraction, interference.
- Electricity and Magnetism: Electric fields, magnetic fields, electromagnetism.
- Optics: Reflection, refraction, diffraction.
- Mathematics: Geometry, vectors, calculus (implied through the dynamic visualizations)
- Other: Including areas like economics, geography, and chemistry.
- Use of Easy JavaScript Simulations (EJS): The simulations are primarily created using the Easy JavaScript Simulations (EJS) modeling tool. EJS allows for the creation of interactive models in JavaScript, making them accessible on various platforms (PCs, tablets, smartphones) through web browsers.
- Quote: "Although EJS is a Java program, EJS 5 creates stand alone JavaScript programs that run in almost any PC or tablet browser."
- Open Educational Resources: The platform is a repository of Open Educational Resources (OER). This means the simulations and related materials are freely available for educational use under a Creative Commons license, encouraging adaptation, modification, and sharing.
- Emphasis on Visualization: The simulations focus heavily on visualizing abstract concepts, enabling students to see and understand the underlying processes. For example, the molecular dynamics simulations allow students to observe particle interactions and movement.
- Quote: "The model shows a liquid material on the left (small atoms). The amount of heat energy is shown by kinetic energy (KE) shading, with deeper shades of red representing more energetic atoms."
- Adaptable for Different Educational Levels: The resources are categorized for different educational levels (Primary, Secondary, Junior College), making it easier for educators to find appropriate material for their students.
- Computational Performance Testing: The "Molecular Dynamics JavaScript Performance Model" was designed to test the computational speed of JavaScript for intensive models. This suggests a potential use case for testing computational performance and limitations.
- Integration of Multiple Technologies: Simulations are provided in formats including HTML5 applets, JavaScript, and WebGL.
- Integration with Student Learning Space (SLS): The platform includes simulations designed for integration with the Student Learning Space (SLS), a digital learning platform in Singapore. There is also emphasis on using xAPI for learning analytics.
- Quote: "Creating HTML5 Content for Interactive Response for SLS Integrating xAPI with EJS Simulations: A Step-by-Step Guide"
- Focus on Inquiry-Based Learning: The simulations can be used as a tool for inquiry-based learning, where students can explore concepts, develop hypotheses, and test their ideas through simulations.
- Community and Collaboration: The platform seems to be a result of collaboration between educators and developers, and it encourages sharing and adaptation of simulations.
Specific Simulation Examples Highlighted:
- Molecular Dynamics Model: A key resource that allows users to explore particle interactions under the Lennard-Jones force. It is used to investigate the speed of JavaScript for computationally intensive tasks.
- States of Matter Simulations: A suite of simulations that allow users to observe how molecules behave in solids, liquids, and gases and how intermolecular attractions and energy input affect the states of matter.
- Simple Harmonic Motion Simulations: Several models for springs and mass systems, examining the relationships between acceleration, position, and time.
- Gravitational Potential Simulations: Models that illustrate concepts related to gravity, escape velocity, and potential energy.
- Various Mechanics Simulations: Resources on projectile motion, collisions, frictional models, and many other topics.
Key Takeaways:
- The Open Educational Resources / Open Source Physics @ Singapore platform is a rich source of interactive simulations for physics and mathematics education.
- The platform emphasizes active learning through dynamic and customizable visualizations.
- The use of EJS makes simulations accessible across platforms, and their open licensing promotes sharing and adaptation.
- The resources are suitable for a wide range of educational levels and can be used to support various teaching methodologies.
Recommendations:
- Educators should explore the simulations available on the platform and integrate them into their lessons.
- Researchers can use the "Molecular Dynamics JavaScript Performance Model" to test computational efficiency.
- Developers can contribute to the platform by building new simulations or improving existing ones using EJS.
- Further explore the many "Project" based simulations which offer a wealth of additional teaching resources.
This briefing document provides a comprehensive overview of the provided source, summarizing the key themes, concepts, and the potential impact of these interactive simulations on education.
Molecular Dynamics and Interactive Simulations Study Guide
Quiz
Instructions: Answer the following questions in 2-3 sentences each.
- What is the primary purpose of the Molecular Dynamics JavaScript Performance Model described in the text?
- What force is used in the Molecular Dynamics JavaScript Performance Model to calculate particle trajectories?
- What are the three user-adjustable parameters of the Molecular Dynamics JavaScript Performance Model?
- According to the text, how can a user stabilize the Molecular Dynamics JavaScript Performance Model if it becomes unstable?
- What is Easy JavaScript Simulations (EJS), and what is its relationship to the Molecular Dynamics JavaScript Performance Model?
- What do the linked Concord Consortium interactive models demonstrate regarding states of matter?
- How does the text explain the relationship between energy input and changes in the state of matter, according to the linked interactive?
- Besides molecular dynamics simulations, what other physics concepts are demonstrated by the linked simulations within this resource?
- What does the text state about the movement of molecules in solids, liquids, and gases, and how can the user explore this through the linked interactives?
- What does the provided text say about the accessibility of the simulations on various devices?
Quiz Answer Key
- The primary purpose of the Molecular Dynamics JavaScript Performance Model is to test the speed of JavaScript for a computationally intensive model by calculating the trajectories of particles interacting with a Lennard-Jones force. This simulation allows users to vary several parameters and see how different calculations impact the performance.
- The Molecular Dynamics JavaScript Performance Model calculates particle trajectories using the Lennard-Jones force, which is a mathematical model that describes the interaction potential between pairs of atoms or molecules. This force influences the particles’ movement in the simulation.
- The three user-adjustable parameters in the Molecular Dynamics JavaScript Performance Model are the number of particles, the number of frames per second, and the number of Verlet steps between frames. These parameters allow users to alter the simulation's complexity and performance.
- If the Molecular Dynamics JavaScript Performance Model becomes unstable, users can stabilize it by resetting the model and reducing the computational timestep (dt). A smaller timestep results in more stable and accurate calculations.
- Easy JavaScript Simulations (EJS) is a modeling tool used to develop the Molecular Dynamics JavaScript Performance Model. EJS creates standalone JavaScript programs that can run in most PC and tablet browsers, even though it's a Java program.
- The linked Concord Consortium interactive models demonstrate the movement of molecules in different states of matter (gas, liquid, solid), showing how molecules move relative to each other. They also illustrate the relationship between intermolecular attractions and the state of matter.
- The linked interactive demonstrates how energy input, represented by kinetic energy (KE) shading, causes matter to change states. By running the model and removing the barrier, users observe that more energetic atoms can melt solids.
- The simulations linked in this resource demonstrate concepts like simple harmonic motion, gravitational fields, collisions, friction, projectile motion, vector addition, wave superposition, and optical diffraction. This demonstrates the large range of physics content that can be modeled using EJS.
- The text states that molecules in all states of matter are in constant motion. Users can explore how this movement varies between solids, liquids, and gases by using the linked interactive models and marking two atoms to observe their relative movement.
- The text mentions that the models are designed to be accessible on various devices including Android/iOS handphones/tablets/iPads, Windows/MacOSX/Linux laptops/desktops, and Chromebook laptops, indicating cross-platform compatibility.
Essay Questions
Instructions: Answer the following questions in a well-organized essay.
- Discuss the role of simulations in learning about physics, using examples from the provided text. How do interactive elements enhance the learning experience compared to traditional methods?
- Analyze the purpose and function of the Molecular Dynamics JavaScript Performance Model. How does it test JavaScript speed, and what are the key parameters that users can manipulate to do so?
- Describe the various physics concepts covered in the linked simulations, and discuss how these interactive models contribute to a deeper understanding of the underlying principles in these domains.
- Compare and contrast the movement of molecules in solids, liquids, and gases according to the provided text, and explain how users can explore these concepts through the interactive linked resources.
- Examine the role of Easy JavaScript Simulations (EJS) as a tool for creating educational resources and discuss its impact on the accessibility and interactivity of physics simulations for learners.
Glossary
- Molecular Dynamics: A computer simulation method for studying the physical movements of atoms and molecules.
- Lennard-Jones Force: A mathematical model describing the interaction potential between pairs of neutral atoms or molecules.
- JavaScript: A programming language used to create interactive effects within web browsers.
- Frames Per Second (FPS): The rate at which consecutive images, called frames, appear in a display and contribute to smooth animation.
- Verlet Algorithm: A numerical method used to integrate equations of motion to compute the trajectories of particles.
- Computational Timestep (dt): The time interval between successive calculations in a simulation; a smaller dt increases accuracy but requires more processing time.
- Easy JavaScript Simulations (EJS): A modeling tool used to develop interactive simulations, capable of creating standalone JavaScript programs.
- Intermolecular Attractions: The forces that act between molecules, influencing the physical state of matter (solid, liquid, gas).
Additional resources
Amazing html5 applet
http://physics.weber.edu/schroeder/md/
http://www.harcourtschool.com/activity/states_of_matter/molecules.swf
https://interactives.ck12.org/simulations/chemistry/states-of-matter/app/index.html?screen=sandbox
Explore how molecules in a gas move relative to each other.
Molecules are in constant motion. "Mark two atoms," then run the model to see how the molecules move relative to each other. How do the distances between gas molecules change over time?
https://lab.concord.org/embeddable.html#interactives/sam/phase-change/2-two-types-of-gases.json
Explore how molecules in a liquid move.
Molecules are in constant motion. "Mark two atoms," then run the model to see how the molecules in a liquid move relative to each other. How does the movement of molecules explain why liquids take the shape of their containers?
https://lab.concord.org/embeddable.html#interactives/sam/phase-change/3-liquids.json
What does a solid look like at the molecular level?
Molecules are in constant motion—even those in a solid! "Mark two atoms," then run the model to see how the molecules in a solid move relative to each other. How would you describe the movement and arrangement of molecules in a solid?
https://lab.concord.org/embeddable.html#interactives/sam/phase-change/4-solids.json
Explore how states of matter are related to the strength of intermolecular attractions.
There are three states of matter—solid, liquid and gas. Run the model and change the strength of attractions between the molecules. How does changing the force of attraction between molecules affect the state of that material?
Explore how energy input causes matter to change states.
Matter exists as solids, liquids and gases, and can change state between these.
The model shows a liquid material on the left (small atoms). The amount of heat energy is shown by kinetic energy (KE) shading, with deeper shades of red representing more energetic atoms. On the right side of the barrier is a solid material (large atoms).
Run the model. How much energy is able to penetrate the barrier? Remove the barrier. How quickly do the more energetic atoms melt the solid?
Frequently Asked Questions About the Physics Simulations
- What is the "Super Powerful Simulator" and what kinds of simulations does it offer?
- The "Super Powerful Simulator" is a collection of interactive physics simulations developed using Easy JavaScript Simulations (EJS). It allows users to explore a wide range of physical phenomena, including:
- Molecular dynamics (two atoms, diatomic gas) using the Lennard-Jones force.
- Classical mechanics (bouncing ball, chaotic bouncers, nano-pinball, slingshot, projectile motion)
- Thermal physics (Brownian motion, hot and cold interactions)
- Wave phenomena (plucked string)
- Explosions
- Friction
- Target and meteor interactions
- And much more, including simulations related to simple harmonic motion, gravity, electromagnetism, and optics. The simulator supports various devices (desktops, laptops, tablets, smartphones) and browsers, making it accessible for diverse learning environments.
- What is the Molecular Dynamics JavaScript Performance Model and what are its main features?
- The Molecular Dynamics JavaScript Performance Model is a simulation designed to test the computational capabilities of JavaScript. It simulates the motion of particles interacting through a Lennard-Jones force and allows users to:
- Vary the number of particles.
- Adjust the frame rate.
- Control the number of Verlet steps per frame.
- Adjust the time step (dt) to maintain simulation stability. This tool is useful for understanding the trade-offs between simulation accuracy and computational speed.
- How can I use the simulations to understand the states of matter (solid, liquid, gas) at the molecular level?
- The simulations include interactive models that explore the behavior of molecules in different states of matter. You can:
- Track the movement of individual molecules ("Mark two atoms") in gases, liquids, and solids.
- Observe how the distances between gas molecules change over time.
- Analyze how the movement of molecules explains why liquids take the shape of their containers.
- Examine the arrangements and movements of molecules in solids.
- Change the strength of attraction between molecules to see how the state of the material changes. These simulations help visualize the microscopic differences between the phases of matter.
- What is Easy JavaScript Simulations (EJS), and how are these simulations developed?
- Easy JavaScript Simulations (EJS) is a modeling tool that enables developers to create interactive simulations that run in web browsers. Although EJS is primarily a Java program, it can produce standalone JavaScript programs that run on most computers and devices. These simulations are primarily built in javascript, which can then be used on browsers using HTML. EJS is used to build the Molecular Dynamics Performance model and many of the other simulations on this page.
- What are some of the physics concepts that can be explored using these simulations besides states of matter? The simulations cover a broad range of topics in physics, including:
- Mechanics: Simple harmonic motion (spring-mass systems, pendulums), gravitational forces, projectile motion, collisions, friction.
- Electromagnetism: Electric fields, magnetic fields, electromagnetic induction.
- Optics: Diffraction, single slit, double slit.
- Modern Physics: Special Relativity, Hydrogen atom.
- Waves: Wave superposition
- Thermodynamics: Heat transfer and kinetic energy of particles.
- Are these simulations suitable for different educational levels?
- Yes, the simulations are designed for a range of educational levels, from primary to junior college. Many of the simulations are appropriate for secondary level learning as well. The resource makes special note of simulations applicable to primary, secondary, and junior college, although many simulations can be used for multiple educational levels. They can be adapted for a variety of learning environments using devices from a range of computers to phones.
- How do the simulations integrate with other learning resources?
- These simulations are intended to enhance learning and may be used with other educational tools and platforms. The resource makes note of how it connects with the Singapore Student Learning Space (SLS), xAPI, and other media resources. By connecting these simulations with different platforms and learning management systems, a more thorough learning experience can be created.
- Where can I find more information about the tools used to create these simulations?
- More information about Easy JavaScript Simulations (EJS) is available at http://www.um.es/fem/Ejs/ and the OSP ComPADRE collection at http://www.compadre.org/OSP/. These resources will provide additional details about the modeling tool, its capabilities, and related open-source physics projects.
- Details
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 12972