About
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits


 Fu-Kwun Hwang; Francisco (Paco) Esquembre; lookang
Fu-Kwun Hwang; Francisco (Paco) Esquembre; lookang
Briefing Document:⚛️ Ideal Gas Model & Kinetic Theory Simulation
1. Overview
This document analyzes a resource from Open Educational Resources / Open Source Physics @ Singapore that provides an interactive simulation of the ideal gas model based on the kinetic theory of gases. The resource aims to enhance understanding of gas behavior by visualizing molecular interactions and their effects on pressure, volume, and temperature. The simulation, built using Easy JavaScript Simulation (Ejs), allows users to manipulate variables and observe the corresponding changes, facilitating a hands-on learning experience.
2. Core Concepts and Themes
- Kinetic Theory of Gases: The foundation of this resource is the kinetic theory, which explains gas behavior based on the motion of its constituent particles. The simulation embodies this by showing particles in random motion, colliding with each other and the container walls.
- Ideal Gas Assumptions: The simulation is based on several key assumptions of the ideal gas model:
- "The molecules in the gas can be considered small hard spheres." This simplifies the representation of gas molecules.
- "All collisions between gas molecules are elastic and all motion is frictionless (no energy is lost in collisions or in motion)." This means that kinetic energy is conserved in interactions.
- "Newton’s laws apply." This ensures that the simulation is based on established physical principles.
- "The distance between molecules on average is much larger than the size of the molecules." This assumption is essential for the ideal gas model to hold true.
- "The gas molecules are constantly moving in random directions with a distribution of speeds." This illustrates the dynamic nature of gas particles.
- "There are no attractive or repulsive forces between the molecules or the surroundings." This simplifies the interactions by focusing solely on collisions.
- Visualizing Gas Pressure: The simulation directly demonstrates how gas pressure arises from the collisions of molecules with the container walls: "What gave rise to a gas pressure was the collisions of the air molecules with the walls of the container containing the air." It further explains that "The force per unit area gave rise to the pressure exerted by the molecules on the walls of the container."
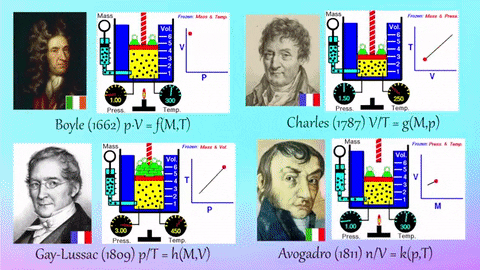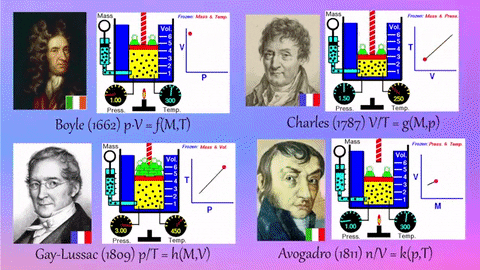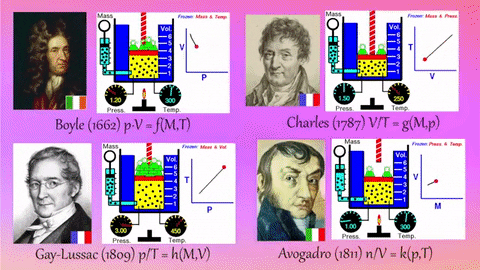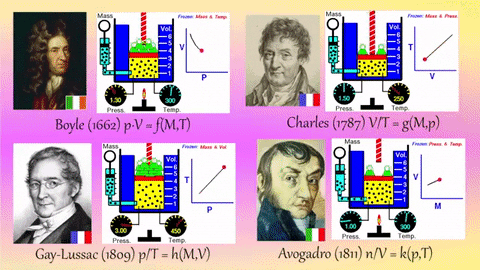
- Relationships Between Gas Properties: The resource explores fundamental relationships between pressure, volume, temperature, and number of gas molecules, specifically focusing on:
- Isothermal Process (Constant Temperature): How changes in volume affect pressure. "What happens to the pressure when the volume changes?" The simulation shows that as volume decreases, pressure increases, and vice-versa.
- Isobaric Process (Constant Pressure): How changes in temperature affect volume. The resource poses the question, "Why does the barrier move when the temperature changes?" The simulation illustrates that at constant pressure, volume expands with increasing temperature and contracts with decreasing temperature.
- Isochoric Process (Constant Volume): How changes in temperature affect pressure. The simulation allows the user to observe, "What happens to the pressure when the temperature changes?" At constant volume, an increase in temperature results in an increase in pressure.
- Relationship between Number of Molecules and Volume: The simulation also demonstrates how volume is related to the number of gas molecules, showing that under constant pressure, increasing the number of molecules increases the volume and vice-versa. "What is the relationship between the number of molecules and the volume of a gas?"
- Interactive Learning: The resource emphasizes a hands-on, interactive approach where students can explore the relationships between variables by actively manipulating the simulation. This allows for a deeper and more intuitive understanding of gas behavior. The learning process is structured through individual and group exploration, promoting active participation.
3. Key Activities & Pedagogical Approach
- Prior Knowledge Activation: The lesson starts by connecting to earlier concepts of pressure, preparing students for the new ideas.
- Trigger Activity: The inflation of balloons is used as a relatable trigger, making the concept of pressure tangible.
- Analogy Activity: Using a student as a "wall" and a ping pong ball as an "air molecule" provides an accessible analogy of how collisions create pressure.
- Exploration Activity: The core of the lesson is centered on interactive simulations where students explore how altering parameters affects gas behavior. This involves both individual observations and group discussions to explain the molecular level mechanisms behind the changes.
- Facilitated Learning: The teacher facilitates the learning process by correcting and adding to student explanations.
4. Simulation Details & Functionality
- Ejs-Based Simulation: The simulation is built using Easy Java Simulation (Ejs), making it accessible on various platforms (Windows, MacOSX, Linux, Android, iOS).
- Variable Manipulation: Users can adjust parameters like velocity, volume, temperature, and number of particles using slider bars and dropdown menus.
- Pressure Gauge: The simulation visualizes pressure by showing how the container wall moves (acting as a pressure gauge), due to molecular collisions.
- Graphing capabilities: The simulation also offers additional options for p-V, p-T, and V-T graphs, allowing students to visualize these relationships directly.
- Embedded Simulations: The document includes direct links to several embedded simulations:
- Volume-pressure relationship.
- Temperature-volume relationship.
- Temperature-pressure relationship.
- Number of molecules-volume relationship.
5. Additional Resources The resource provides several links to external resources including:
- Gas Property Lab by PhET
- Flipped Video Lectures by Andreas Dewanto on the Ideal Gas Equation and Kinetic Theory.
6. Conclusion This resource offers a comprehensive and interactive approach to understanding the ideal gas model based on the kinetic theory. The interactive simulations, coupled with a thoughtful pedagogical approach, allow students to explore gas behavior actively. This allows for a more intuitive and deeper understanding of the microscopic processes that underlie the macroscopic behavior of gases.
Ideal Gas Model Study Guide
Quiz
Instructions: Answer the following questions in 2-3 sentences each.
- According to the ideal gas model, what are the physical characteristics of the gas molecules themselves?
- What type of collisions do gas molecules experience, according to the ideal gas model, and what is conserved in these collisions?
- Briefly explain the analogy activity used in the lesson concerning the wall of a container and a ping pong ball.
- How does increasing the temperature of a gas affect its pressure if the volume is held constant?
- How does decreasing the volume of a gas affect its pressure if the temperature is held constant?
- In the models, the atoms are in a flat plane. How is the volume of the container still calculated?
- What happens to the volume of a gas if you increase the temperature while maintaining a constant pressure?
- What is the relationship between the number of gas molecules and the volume of the gas when pressure is constant?
- What role does the "moving wall" play in the simulations, and how does it relate to pressure?
- What are two ways that the simulations allow the user to change the parameters of the gas being modeled?
Quiz Answer Key
- The gas molecules are considered small, hard spheres, and the distance between molecules on average is much larger than the size of the molecules. These molecules do not have internal attractive or repulsive forces between each other.
- The ideal gas model assumes that all collisions between gas molecules are elastic, which means that kinetic energy is conserved during collisions. No energy is lost to friction or other non-conservative forces.
- The analogy uses a student representing a container wall and a ping pong ball representing a gas molecule. The impact of the ball on the student shows how gas molecules exert force on the container walls, creating pressure.
- Increasing the temperature of a gas increases the kinetic energy of the molecules, causing them to collide with the container walls more forcefully and frequently, leading to a higher pressure. This is under conditions of constant volume.
- Decreasing the volume of a gas while maintaining constant temperature will increase the gas pressure because the molecules collide with the container walls more frequently. There is less space in which for the molecules to move freely.
- Although the atoms are in a flat plane in the model, volume is still calculated by using a depth of 0.1 nm, effectively making the simulation three-dimensional when calculating volume.
- If the temperature of a gas increases while pressure is kept constant, the volume of the gas increases. This is because the molecules move faster, requiring more space to maintain the constant pressure.
- When the pressure is constant, the volume of a gas is directly proportional to the number of gas molecules. More molecules occupy more volume, and fewer molecules occupy less volume.
- The "moving wall" acts as a pressure gauge, converting the impact of molecular collisions into a visible representation of the gas's pressure. It also can adjust the volume of the simulation.
- The user can change the velocity and pressure using a slider bar. The user can also select different numbers of molecules from a drop-down menu, observing how the number of molecules impacts the volume.
Essay Questions
Instructions: Answer the following essay questions using information derived from the provided source material.
- Discuss the assumptions of the ideal gas model, and explain how these assumptions lead to the observed relationships between pressure, volume, and temperature.
- Describe the experimental setup and methodology used in the provided simulations to explore the relationships between temperature, pressure, and volume. Discuss how the simulations illustrate the effects of changing each of these parameters.
- Compare the real-world process of inflating a balloon with the model of ideal gas behavior. What are some factors that the ideal gas model does not account for in the real-world example?
- Explain how the analogy activity involving the ping pong ball and a student helps to illustrate the concept of gas pressure. What are its strengths and limitations as an educational tool?
- How do the simulation exercises relate to the overall goal of teaching about ideal gases and the kinetic theory of gases?
Glossary of Key Terms
Ideal Gas Model: A simplified model of gas behavior that assumes gas molecules are small, hard spheres, do not attract or repel each other, and collide elastically.
Kinetic Theory of Gases: A theory explaining the macroscopic properties of gases in terms of the motion of their constituent particles.
Elastic Collision: A collision where kinetic energy is conserved, meaning no energy is lost to other forms like heat or sound.
Pressure: The force exerted per unit area, often measured in Pascals (Pa). In a gas, pressure is the result of collisions between gas molecules and the walls of their container.
Temperature: A measure of the average kinetic energy of the particles within a substance, often measured in Kelvin (K). Higher temperatures indicate faster moving particles.
Volume: The amount of space that a substance occupies, typically measured in cubic meters (m³) or liters (L). In a simulation, this is the size of the container holding the gas.
Isothermal Process: A thermodynamic process where the temperature of the system remains constant.
Isobaric Process: A thermodynamic process where the pressure of the system remains constant.
Isochoric Process: A thermodynamic process where the volume of the system remains constant.
Kinetic Energy: The energy an object has due to its motion, given by the formula 1/2 * mv², where m is mass and v is velocity.
Learning Outcomes
- the variation of gas pressure with temperature (Volume is constant)
- the variation of gas pressure with volume (Temperature is constant)
- the variation of the volume of gas with temperature (Pressure is constant)
Prior Knowledge Activation
Teacher active prior knowledge by reminding students that earlier in Chapter 7, they had studied about pressure and in today’s lesson, they were going to take the concept of pressure further to understand what gave rise to a gas pressure.
Trigger Activity
A trigger activity, the teacher had asked for two volunteers to inflate two balloons. After the balloons were inflated, students were told that the balloons could also had been blown up using a hand or foot pump. A picture of a foot pump with a pressure gauge was shown to the students, and the teacher suggests if it is possible that the balloon had been inflated using the pump, the pressure gauge would register a pressure reading, and would that be similar to the case where car tyres were pumped at petrol kiosks and the pressure meters would read the pressure of the air in the tyres?
Analogy Activity
Analogy activity was conducted through another student volunteer where the student would represent the wall of the container and a ping pong ball would represent an air molecule. The ping pong ball was gently thrown at the student’s body, and the student experienced an impact, and hence experienced a force. Now if the ball was thrown at the student with greater speed, the impact would be greater and the force would be greater. It was then explained to students that what gave rise to a gas pressure was the collisions of the air molecules with the walls of the container containing the air. As there were numerous collisions between the air molecules and the wall, an average force was exerted on the wall. The force per unit area gave rise to the pressure exerted by the molecules on the walls of the container.
Exploration Activity
In the next part of the lesson, the teacher assigned each group to look into a specific exploration task . Within each task, there was a segment for individual exploration where the students followed the instructions on the worksheet to make individual observations on how changing certain parameters of temperature, pressure or volume would change other parameters, and a segment for group exploration where the students would come together and use their individual findings to explain in molecular terms the how and why of those changes. The group would then share their explanations online. The group then nominated a member to present their answers to the class, and the teacher would facilitate the learning by correcting the explanations, as well as adding on to the answers.
Other Related Resources
- http://phet.colorado.edu/en/simulation/legacy/gas-properties Gas Property Lab by PhET
- Flipped Video Lecture 11.1 Ideal Gas Equation by Andreas Dewanto
- Lecture 11.2 Kinetic Theory Of Ideal Gas by Andreas Dewanto
- Lecture11 3 WorkByIdealGas by Andreas Dewanto
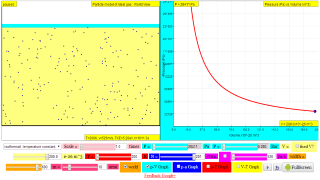
Explore how the volume of a gas affects pressure, (constant temperature, isothermal).
Gases can be compressed into smaller volumes. How does compressing a gas affect its pressure?
Run the model, then change the volume of the containers and observe the change in pressure. The moving wall converts the effect of molecular collisions into pressure and acts as a pressure gauge. What happens to the pressure when the volume changes?
Note: Although the atoms in this model are in a flat plane, volume is calculated using 0.1 nm as the depth of the container.
http://lab.concord.org/embeddable.html#interactives/sam/gas-laws/3-volume-pressure-relationship.json
Investigate the relationship between temperature and the volume of a gas, (constant pressure, isobaric)
This model contains gas molecules on the left side and a barrier that moves when the volume of gas expands or contracts, keeping the pressure constant. Run the model and change the temperature. Why does the barrier move when the temperature changes?
Note: Although the atoms in this model are in a flat plane, volume is calculated using 0.1 nm as the depth of the container.
Consider how temperature affects the pressure exerted by a gas, constant volume, isochoric)
Run the model and change the temperature. What happens to the pressure when the temperature changes?
Explore how the volume of a gas is related to the number of gas molecules.
The model contains gas molecules under constant pressure. The barrier moves when the volume of gas expands or contracts. Run the model and select different numbers of molecules from the drop-down menu. What is the relationship between the number of molecules and the volume of a gas?
Note: Although the atoms in this model are in a flat plane, volume is calculated using 0.1 nm as the depth of the container.
http://lab.concord.org/embeddable.html#interactives/sam/gas-laws/6-number-volume-relationship.json
FAQ: Understanding the Ideal Gas Model and Kinetic Theory
- What are the key assumptions of the ideal gas model according to the kinetic theory?
- The ideal gas model makes several key assumptions: (1) Gas molecules are considered small, hard spheres. (2) All collisions between gas molecules and with the container walls are perfectly elastic (no energy is lost). (3) Newton's laws of motion apply to the molecules. (4) The average distance between molecules is much larger than their size. (5) Molecules are in constant, random motion with a range of speeds. (6) There are no attractive or repulsive forces between molecules or between the molecules and the container. These assumptions simplify the model to help us understand gas behavior.
- How does the kinetic theory explain gas pressure?
- Gas pressure arises from the countless collisions of gas molecules with the walls of their container. Each collision exerts a tiny force on the wall. The sum of these tiny forces over the area of the wall creates an overall pressure. The more frequent and more forceful the collisions, the greater the gas pressure. This is why increasing temperature (which leads to faster particle movement) or decreasing volume (which leads to more frequent collisions) increases pressure.
- How does changing the volume of a gas affect its pressure, assuming a constant temperature?
- When the volume of a gas is decreased while keeping the temperature constant, the pressure increases. This is because compressing the gas reduces the space that the gas molecules have to move around in. This leads to more frequent collisions with the walls of the container, therefore increasing the force per unit area and consequently, the pressure. Conversely, expanding the gas volume would decrease the pressure for the same reason.
- What happens to the volume of a gas when its temperature is increased, assuming a constant pressure?
- When the temperature of a gas is increased while keeping the pressure constant, the volume of the gas will also increase. The increase in temperature means the gas molecules are moving faster, leading to more forceful collisions with the walls. To maintain constant pressure, the volume must expand so that the frequency of collisions per unit area decreases to compensate for the increased force of the collisions. This way the pressure exerted by the gas on the container remains the same.
- How does changing temperature affect gas pressure when volume is kept constant?
- When temperature is increased at constant volume, the gas pressure increases. This is because a higher temperature results in the gas molecules moving at higher speeds. This leads to more forceful collisions with the container walls, which results in an increase in the average force per unit area and therefore increases gas pressure. The molecules colliding more often will also increase the pressure.
- How does the number of gas molecules affect volume, assuming constant pressure and temperature?
When the number of gas molecules is increased at constant pressure and temperature, the volume also increases. The addition of molecules increases the total number of collisions with the container's walls. To maintain constant pressure, the container volume must expand, allowing the number of molecules colliding per unit area of the walls to remain the same. This is why inflating a balloon expands it, and it's also why gas volume is proportional to the number of molecules in an ideal gas.
- What are some practical demonstrations that show how gas pressure and volume are related?
- The source gives some examples that demonstrate gas pressure and volume: The process of inflating a balloon using a hand pump or foot pump illustrates that increasing the amount of gas into a fixed volume (the balloon) increases pressure within. Inflating car tires similarly demonstrates that pumping more air into the tires increases their pressure. These are real-world examples of gas dynamics from the kinetic theory of gases.
- Are there interactive simulation tools mentioned to help visualise the ideal gas model?
- Yes, the source mentions several interactive simulation tools. Specifically, it uses java-based simulations and links to others such as, "Gas Property Lab by PhET" and models that use WebEJS. These simulations help students explore the relationships between pressure, volume, temperature, and number of molecules in a gas, which makes them a useful way to grasp the concepts behind the ideal gas law and kinetic theory. These simulations may offer features to change elasticity, p-V, p-T and V-T graphs.
- Details
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 15448