Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits



 Written by Loo Kang Wee; Felix J. Garcia Clemente; Francisco Esquembre; Designed by David Loh
Written by Loo Kang Wee; Felix J. Garcia Clemente; Francisco Esquembre; Designed by David Loh
1. Introduction:
This briefing document reviews two closely related online resources focused on teaching and learning about covalent bonding using 'dot and cross' diagrams. Both resources are simulations designed to help students visualize the sharing of electrons in covalent bonds. They share the same authors and designers, indicating a cohesive educational tool. This review will highlight the main themes, important ideas, and student feedback related to these simulations.
2. Main Themes and Important Ideas:
- Interactive Learning of Covalent Bonding: The central theme is the use of interactive JavaScript/HTML5 simulations to teach the concept of covalent bonding and how to represent it using dot and cross diagrams. This approach emphasizes "learning by doing" and provides immediate feedback to students.
- Visualization of Electron Sharing: The simulations allow students to manipulate electrons (represented as dots and crosses for different atoms) and place them in the electron shells of atoms to form covalent bonds. This visual representation aims to make the abstract concept of electron sharing more concrete and understandable. As one student noted, "It was helpful to visualise the concepts."
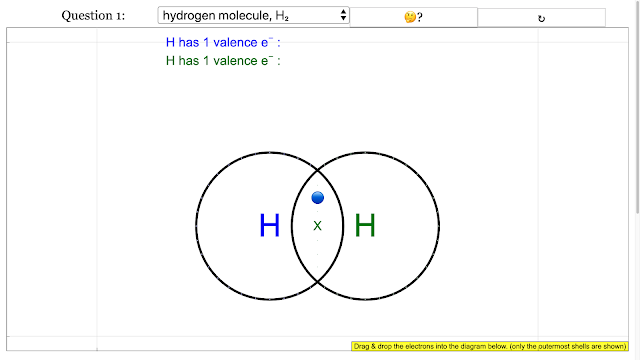
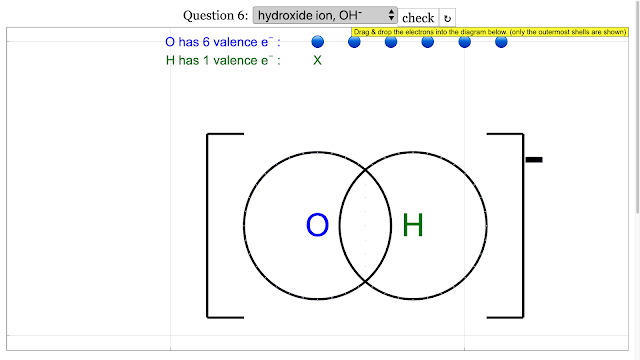
- Focus on Key Molecular Structures: The resources provide specific examples of dot and cross diagrams for various molecules and polyatomic ions, including hydrogen (H₂), chlorine (Cl₂), oxygen (O₂), nitrogen (N₂), hydrogen chloride (HCl), hydroxide ion (OH⁻), cyanide ion (CN⁻), water (H₂O), carbon dioxide (CO₂), nitrite ion (NO₂⁻), ammonia (NH₃), methane (CH₄), carbonate ion (CO₃²⁻), and hydrogen peroxide (H₂O₂). For each example, the resources often provide the "answer," which indicates the correct arrangement of electrons to achieve stable electron configurations (usually a duet for hydrogen and an octet for other atoms in the outermost shell). For example, for the Hydrogen molecule, the answer is "add up to 2 on each H atom electron outermost with shared electrons = 2."
- Accessibility and Embeddability: The simulations are designed to be accessible through web browsers using HTML5, making them usable on various devices, including Chromebooks. The "Embed" option provides an iframe code, allowing teachers to easily integrate the simulation into their own webpages or learning management systems.
- Emphasis on Self-Learning and Immediate Feedback: Student feedback highlights the value of being able to "try it out ourselves" and receive immediate feedback. One student appreciated that the simulation "allowed me to understand more about the formation of covalent bonds." Another noted, "It was a lot easier to correct small errors using the simulation that it is with pen and paper."
- Teacher Resources and Data Collection: The resources include links to Google Slides for teachers and encourage Singaporean teachers to contact the developers for a digital Google survey form to collect anonymous data on the effectiveness of the lesson. This indicates a commitment to ongoing improvement based on classroom usage.
- Positive Student Reception: The "Student Survey" section provides overwhelmingly positive feedback regarding the effectiveness, engagement, and ease of use of the simulation. Many students found it more interesting and helpful than traditional methods like drawing diagrams on paper. Quotes like "The simulation made the lesson more interesting as it was interactive" and "The simulation helps me to understand better about chemical bonding dot & cross diagram" are representative of this sentiment.
- Areas for Improvement Identified by Students: While the feedback is largely positive, students also offered suggestions for improvement, including making the dragging of electrons less tedious, adding more visual appeal, incorporating explanations for incorrect answers, offering a wider range of compounds, and improving usability for non-touchscreen devices. The developers have responded to some of this feedback, such as increasing the sensitivity of the electron dragging area and considering a video tutorial.
3. Key Facts and Information:
- Authorship and Design: The simulations are written by Loo Kang Wee, Felix J. Garcia Clemente, and Francisco Esquembre, and designed by David Loh.
- Licensing: The resources are released under a Creative Commons Attribution-Share Alike-NonCommercial license (CC-BY-SA-NC). The underlying Easy JavaScript Simulations library has a separate commercial use license.
- Target Audience: The simulations are designed for O Level and A Level chemistry students, as indicated by the sample learning goals.
- Platform: The simulations are built using EJS (Easy JavaScript Simulations) and are implemented in JavaScript and HTML5, making them web-based.
- Availability of Examples: The resources provide direct links to simulations for a variety of specific molecules and ions, allowing students to focus on particular examples.
- Evidence of Effectiveness: The developers cite their own survey suggesting "strong evidence on effective student learning, appreciative students, good simulation design." The detailed student feedback provided further supports this claim.
4. Quotes from Original Sources:
- "answer is add up to 2 on each H atom electron outermost with shared electrons = 2" (Example answer for Hydrogen molecule)
- "Our own survey suggests strong evidence on effective student learning, appreciative students, good simulation design."
- Student comments:
- "It was helpful to visualise the concepts."
- "The simulation made the lesson more interesting as it was interactive"
- "The simulation helps me to understand better about chemical bonding dot & cross diagram."
- "It was fun and was an easier and more interesting way to learn about covalent bonding instead of literally drawing the atom over and over again."
- "I liked the fact that we got to learn through using our chromebook and got to try it out ourselves."
- "The simulation allowed me to discover where the electrons should go and somehow, why it was like that."
- "It was a lot easier to correct small errors using the simulation that it is with pen and paper."
- "Dragging and clicking of the electrons became tiring after a while..." (Example of a suggestion for improvement)
- "This app is really interesting. Thank you for spending time to make this app!" (Example of positive feedback)
5. Conclusion:
The "Covalent Bonding 'Dot and Cross' Simulation" and its related resources provide a valuable and engaging tool for teaching and learning about covalent bonding. The interactive nature of the simulations, the visual representation of electron sharing, and the immediate feedback are highly appreciated by students and contribute to effective learning. While students have offered constructive suggestions for improvement, the overall feedback indicates that these simulations are a significant enhancement over traditional methods of teaching this fundamental chemical concept. The developers' commitment to gathering feedback and iteratively improving the resource is also noteworthy.
Study Guide: Covalent Bonding Dot and Cross Diagrams
Key Concepts
- Covalent Bonding: A type of chemical bond formed by the sharing of electron pairs between atoms. This typically occurs between nonmetal atoms.
- Valence Electrons: The electrons in the outermost shell of an atom. These are the electrons involved in chemical bonding.
- Dot and Cross Diagrams: Visual representations of covalent bonds showing the valence electrons of participating atoms. One atom's electrons are usually represented by dots, and the other atom's by crosses. Shared electrons are shown in the overlapping region between the atoms.
- Octet Rule (and Duet Rule): The tendency of atoms to prefer to have eight electrons in their valence shell (an octet), achieving the same electronic configuration as a noble gas. Hydrogen and lithium aim for two electrons (a duet), similar to helium.
- Molecule: A group of two or more atoms held together by covalent bonds.
- Polyatomic Ion: A charged species composed of two or more atoms covalently bonded together. The entire group of atoms carries an overall charge due to an imbalance in the total number of protons and electrons.
- Shared Electrons: The valence electrons that are mutually attracted to the nuclei of both atoms in a covalent bond, effectively holding the atoms together.
- Lone Pair Electrons (Non-bonding Electrons): Valence electrons that are not involved in covalent bonds and are exclusive to a single atom in a molecule or ion.
Review Questions
- What is the fundamental difference between ionic and covalent bonding?
- Why do atoms form covalent bonds? Explain in terms of electron configuration and stability.
- Describe how to draw a dot and cross diagram for a simple molecule like hydrogen (\(H_2\)). What does the diagram represent?
- Explain the significance of the octet rule in the formation of covalent bonds. Are there any exceptions to this rule mentioned in the source?
- How are dot and cross diagrams adapted to represent polyatomic ions, such as hydroxide (\(OH^-\)) or cyanide (\(CN^-\))? What additional information needs to be included?
- What is the relationship between the number of shared electron pairs in a covalent bond and the terms single bond, double bond, and triple bond? Give examples from the provided molecules.
- How does the dot and cross diagram for hydrogen chloride (\(HCl\)) illustrate that one atom achieves an octet while the other achieves a duet?
- Explain how the dot and cross diagrams for oxygen (\(O_2\)) and nitrogen (\(N_2\)) demonstrate the formation of multiple covalent bonds.
- What information about the valence electrons of each atom is essential to know before drawing a dot and cross diagram? How can you determine this information?
- How did the simulation tool described in the source material aid students in learning about dot and cross diagrams for covalent bonding? What aspects of the simulation were particularly helpful?
Quiz
- Describe the process of covalent bond formation. In your explanation, highlight what is being shared and why this sharing occurs.
- Using the example of a chlorine molecule (\(Cl_2\)), explain how a dot and cross diagram illustrates the achievement of a stable electron configuration for each chlorine atom.
- What is a polyatomic ion? How does the dot and cross diagram for a polyatomic ion differ from that of a neutral molecule? Provide an example from the source.
- Explain the concept of shared electrons in a covalent bond. How do these shared electrons contribute to the stability of the molecule?
- Draw a simplified dot and cross diagram for water (\(H_2O\)), indicating the shared electron pairs. Explain how the oxygen and hydrogen atoms achieve stable electron configurations.
- What is the octet rule, and why is it important in understanding covalent bonding? Mention one molecule from the source that follows this rule.
- How does the dot and cross diagram for the nitrogen molecule (\(N_2\)) differ from that of the hydrogen molecule (\(H_2\))? What does this difference indicate about the bond?
- Explain how the simulation applet described in the source helped students visualize covalent bonding. Mention a specific feature that students found helpful.
- Describe the information conveyed by the dots and crosses in a dot and cross diagram. What do they represent for each participating atom?
- How does the dot and cross diagram for the cyanide ion (\(CN^-\)) show that it is an ion and that both carbon and nitrogen achieve a stable electron configuration?
Quiz Answer Key
- Covalent bond formation involves the sharing of valence electron pairs between two atoms, typically nonmetals. This sharing allows each atom to achieve a more stable electron configuration, usually resembling that of a noble gas (octet or duet rule).
- In a chlorine molecule (\(Cl_2\)), each chlorine atom has 7 valence electrons. The dot and cross diagram shows one electron from each chlorine atom being shared, forming a single covalent bond. Each chlorine atom now has access to 8 valence electrons (6 of its own and 2 shared), satisfying the octet rule.
- A polyatomic ion is a charged group of covalently bonded atoms. Its dot and cross diagram shows the valence electrons and the shared pairs, but the entire structure is enclosed in brackets with the overall charge of the ion indicated outside. For example, the hydroxide ion (\(OH^-\)) shows the shared electron pair between oxygen and hydrogen, with an extra electron giving the ion a -1 charge.
- Shared electrons are the valence electrons that are attracted to the nuclei of both atoms participating in a covalent bond. By being shared, these electrons effectively fill the valence shells of both atoms simultaneously, leading to a more stable arrangement with lower energy.
- For water (\(H_2O\)), the dot and cross diagram shows an oxygen atom with 6 valence electrons sharing one electron with each of the two hydrogen atoms (each with 1 valence electron). This results in two single covalent bonds. The oxygen atom effectively has 8 valence electrons (4 lone pair and 4 shared), and each hydrogen atom has 2 (shared), achieving stable configurations.
- The octet rule states that atoms tend to gain, lose, or share electrons to achieve a valence shell with eight electrons, resembling a noble gas. It is important because it predicts the formation and stability of many covalent compounds. Carbon dioxide (\(CO_2\)) is an example where each atom (one carbon and two oxygen) achieves an octet through double covalent bonds.
- The dot and cross diagram for nitrogen (\(N_2\)) shows three shared pairs of electrons between the two nitrogen atoms (each with 5 valence electrons), forming a triple bond. In contrast, hydrogen (\(H_2\)) has only one shared pair, forming a single bond. This difference indicates that the triple bond in nitrogen is stronger and involves more shared electrons.
- The simulation applet allowed students to interactively place dots and crosses representing valence electrons to form covalent bonds. Students found the ability to visualize the electron sharing and the immediate feedback on the correctness of their diagrams particularly helpful in understanding the concept.
- The dots and crosses in a dot and cross diagram represent the valence electrons of the different atoms participating in the covalent bond. Typically, one element's valence electrons are shown as dots, and the other's as crosses, making it easy to track which electrons originated from which atom, especially the shared electrons.
- The dot and cross diagram for the cyanide ion (\(CN^-\)) shows a triple bond between carbon (4 valence electrons) and nitrogen (5 valence electrons), with one extra electron giving the ion a -1 charge. This arrangement results in both carbon and nitrogen having access to 8 valence electrons (an octet), contributing to the ion's stability.
Essay Format Questions
- Discuss the importance of dot and cross diagrams as a visual tool for understanding the formation of covalent bonds. How do these diagrams help to illustrate key concepts such as valence electrons, shared electron pairs, and the octet rule? Consider the benefits and limitations of using this model.
- Compare and contrast the formation of single, double, and triple covalent bonds, using examples from the provided source material (\(H_2\), \(O_2\), \(N_2\)). Explain how the number of shared electron pairs affects the properties of the bond, such as strength and length.
- Explain how the concept of the octet rule governs the formation of covalent bonds in many molecules and polyatomic ions discussed in the source. Discuss any exceptions or deviations from the octet rule that might be inferred from the given examples, and why these exceptions might occur.
- Evaluate the effectiveness of using interactive simulations, such as the one described in the source, as a pedagogical tool for teaching abstract concepts like covalent bonding. Based on the student feedback, what are the key advantages and potential areas for improvement of such tools in chemistry education?
- Analyze the role of valence electrons in the formation of covalent bonds. Using specific examples of molecules and polyatomic ions from the source, explain how the arrangement and sharing of valence electrons lead to the formation of stable chemical species.
https://play.google.com/store/apps/details?id=com.ionicframework.covalentbonding this version has the valency question
Sample Learning Goals
- A Level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5
- O level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5
For Teachers
From Google slides (From David) to interactive https://docs.google.com/presentation/d/1fwutLc-jPc1fUyrxJsps3Fhg6_J9y8AEA4pHJ68gdBw/edit?ts=5dd2086a#slide=id.g5292a6c619_0_96
If you are using this simulation in Singapore Schools, please contact This email address is being protected from spambots. You need JavaScript enabled to view it. to get a digital google survey form to collect anonymous data on the effectiveness of lesson and future imporvement to the simulation!. Thanks in advance.
Our own survey suggests strong evidence on effective student learning, appreciative students, good simulation design. Do you agree? let me know in the comments below! https://weelookang.blogspot.com/2020/02/o-level-chemical-bonding-dot-and-cross.html
Chemical Bonding Dot and Cross Diagrams
Polyatomic ions dot and cross diagram
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen molecule answer is add up to 2 on each H atom electron outermost with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Chlorine molecule |
|
Chemical Bonding Dot and Cross Diagrams for Chlorine molecule answer is add up to 8 on each atom's' electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Oxygen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Oxygen molecule answer is is add up to 8 on each atom's' electron outermost shell with shared electrons = 4 |
|
Chemical Bonding Dot and Cross Diagrams for Nitrogen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Nitrogen molecule answer is is add up to 8 on each atom's' electron outermost shell with shared electrons = 6 |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Chloride molecule |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Chloride molecule answer is is add up to 8 on Cl atom and 2 for H atom and electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Hydroxide ion |
|
Chemical Bonding Dot and Cross Diagrams for Hydroxide ion answer is is add up to 8 on O atom and 2 for H atom and electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Cyanide ion |
|
Chemical Bonding Dot and Cross Diagrams for Cyanide ion answer is is add up to 8 on each atoms and electron outermost shell with shared electrons = 6 |
|
Chemical Bonding Dot and Cross Diagrams for Water molecule |
|
Chemical Bonding Dot and Cross Diagrams for Water molecule answer is is add up to 8 on O atom and 2 on H atoms and electron outermost shell with shared electrons = 2,2 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Carbon Dioxide molecule |
|
Chemical Bonding Dot and Cross Diagrams for Carbon Dioxide molecule answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 4,4 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 2,4 respectively with the foreign electron on the O atom with the shared electrons=2 |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 2,4 respectively with the foreign electron on the O atom with the shared electrons=2 |
|
Chemical Bonding Dot and Cross Diagrams for Ammonia molecule |
|
Chemical Bonding Dot and Cross Diagrams for Ammonia molecule answer is is add up to 8 on N atom and 2 on H atom and the electron outermost shell with shared electrons = 2,2,2 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Methane molecule |
|
Chemical Bonding Dot and Cross Diagrams for Methane molecule answer is is add up to 8 on C atom and 2 on H atom and the electron outermost shell with shared electrons = 2,2,2, respectively |
|
Chemical Bonding Dot and Cross Diagrams for Carbonate ion |
|
Chemical Bonding Dot and Cross Diagrams for Carbonate ion answer is is add up to 8 on each atom and the electron outermost shell with shared electrons = 4,2,2, respectively. The two O atoms with 2 shared electrons has a foreign electron in it's personal shell. |
 |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Peroxide |
Student Survey
survey 1 , 2 data from MGS 2020 total 1 + 2 classes estimated number of students (44 , 46) responses.
I have added some answers to the questions asked too.
 |
| typical classroom setting where the anonymous (photo blurred) teacher skillfully explains the learning tasks using https://sg.iwant2study.org/ospsg/index.php/922 |
 |
| typical classroom setting where the anonymous students are each working on their ChromeBook to learn by doing chemistry. this is far better than students working on Google Slides and trying to fit in the electrons on non simulation medium, only getting feedback on the accuracy after the teacher manually marked each students' assigned google slide prepared by the teacher. https://sg.iwant2study.org/ospsg/index.php/922 |
What do you like about the lesson? (For example, was the lesson successful in helping you to learn the concepts? In what way was the simulation helpful? Did it arouse your interest? Please give more details.)
- It was successful in helping me learn the basic concepts but it did not give me a very clear understanding of the concept. I was still left with questions and doubts. It was a unique and new way of learning in the classroom. noted, Video Tutorial will be ready after march holidays.
- Something new. No need to check the periodic table. There are other compounds not tested available. The emoji is cute.
- It was helpful to visualise the concepts.
- the lesson was successful in helping me to learn the concepts. the simulation was helpful. It arouse my interest.
- it is interesting and can help me learn independently.
- The lesson was more hands on than just the teacher explaining to us how to draw dot and cross diagrams
- This is a more interesting style of learning
- The simulation made the lesson more interesting as it was interactive
- the simulation was easy to use and easy to understand
- The simulation helps me to understand better about chemical bonding dot & cross diagram. I also can engage with the lesson better because we get to use technology during lessons and there are colours.
- yes,it helps me a lot ,I was confused about the definition yesterday night ,but through this lesson, I think I know better about the definition and the process .
- it helped me to understand better
- the simulation made me more interested in learning about chemical bonding.
- I like the fact that we got to learn through using our chromebook and got to try it out ourselves.
- it allowed me to understand more about the formation of covalent bonds
- I was fun and was an easier and more interesting way to learn about covalent bonding instead of literally drawing the atom over and over again.
- It keeps my interest because it is easy to use
- it was interesting i guess
- It was interesting and helped me to be more confident in dot and cross diagrams.
- that we get to attempt it ourselves first
- It is easy to use and informative
- it was easy to understand with answers and hints provided
- The simulation was cute and captured my attention so I enjoyed doing it.
- The simulation allowed me to discover where the electrons should go and somehow, why it was like that.
- it's easier to use
- it was easy and fun to use
- The lesson was successful in helping me learn the concepts. It gave me more practice in covalent bonding.
- the simulation gave me a concept of what covalent bonding is about
- The simulation is much easier than drawing the atoms out.
- I like that the lesson allowed me to learn independently and the diagrams were simple and easy to read.
- The lesson helped me have a clear visual on covalent bonding. Helped it to be clearer to me.
- The answers are not given on the first wrong attempt, we can continue to try to get to the correct answer.
- The simulation made the locations of the electrons clearer
- I like that it gives the number of electrons that the user has to use and that the user does not need to refer to the periodic table to do this simulation, increasing interest and ease in doing this while learning something.
- It was a refreshing change. Instead of drawing the diagrams on paper, I could move the electrons online.
- it is very entertaining and educative
- the visuals helped me!
- it was interesting
- The colour coding makes the visuals much more easier to see and understand the concepts.
- The lesson was very fun and engaging. The simulation allowed me to better understand the concepts.
- It was successful in helping me learn the concepts as it provided a solution and explanation when i didn't know how to do the question.There were also many question which allowed me to try out different questions or different levels of difficulty,allowing me to practice more.
- it was fun
- The hints especially helped me to understand the concept
- I can understand the structures more clearly. was quite fun to drag and drop instead of drawing for once. It's also much more convenient as i can check my work immediately instead of waiting for the teacher to come around
- The simulation was easy to use and was a good reference for doing the worksheet as well !
- the simulation was helpful in giving model answers for me to understand covalent bonding better
- The simulation helped me determine the number of valence electrons so it was easier to draw the dot and cross diagram. The simulation was very fun and easy to use and it was a drag and drop application
- was very successful
- the simulation helped me better understand the topic better and it was engaging
- I liked that it was more engaging and gave us room to think for ourselves while having some guidance from the simulation. The simulation made it easier to trial and error so that I could figure out the bonding by myself.
- it is interactive and clear:)
- It helped me understand the concept of bonding better. It was very interesting and helpful. I would like to do it again in class sometime.
- the lesson was easy to follow since the simulation was easy to use and we could just reset instead of redrawing
- It was a lot easier to correct small errors using the simulation that it is with pen and paper.
- It was pretty interactive and it helped us to apply important concepts when drawing dot-cross diagrams.
- - easy to use
- - I had more confidence in drawing dot and cross diagrams
- - wide range of ions to choose from
- - "hints" feature
- It was easy to use and a suitable amount of help was given (number of valence electrons per element were given)
- i liked how it was not too complicated to do the dot-and-cross diagrams and the website was easy to use.
- helpful - it prompted us and gave hints on how to draw the diagram
- yes i am interested
- it was good to check whether my diagrams were right
- Yes it was very helpful. I learnt how to draw polyatomic ions very quickly and I find this kind of simulation useful as I can self-learn without relying so much on the teacher. :)
- It gives a better visualisation of the dot and cross diagram and helps me to be able to draw the diagram easily.
- It made the process of thinking how to draw it much easier
- i was able to learn the concept faster with the use of the website
- It helped me visualise the covalent bonding between the molecules and made me curious to find out why the molecules bonded in a particular way
- I liked using the simulation to trial and error with the diagram and to fill in the worksheet.
- the platform made learning the dot cross diagram more interesting
- it helped me figure out the answer in my own.
- the lesson helped to make my understanding of covalent bonding clearer
- I liked the idea of figuring it out yourself and then having a check answer button this app is great!
- It made visualising the covalent bonds and the orientation of electrons easier.
- ー
- it is fun to drag the electrons around
- it gives me many chances for trial and error without having to continuously erase my mistakes
- I like that we get to explore the concept ourselves and learn by trial and error.
- The simulation was easy to use and allowed me to understand how to draw dot and cross diagrams as it told me what was wrong when I made a mistake
- I learnt how foreign electrons worked, and it was interesting to see try doing the different questions. It was also easier as the simulation already had the electrons shells put out for you.
- It helped me to visualise the concept better
- I liked the lesson because it was more engaging and hands-on, and it also helped me to learn the concepts. It is also better than having to draw out a dot and cross diagram myself.
- It helped me to see the chemical bonds visually which was very helpful
- It allowed me to easily move each electron around before writing it on my paper thus it was easier and neater. Furthermore, because of the difference colours it is easier to see the electrons
- The simulation made it easier for me to visualise the atoms, aiding me in figuring out the configuration of different compounds.
- It was successful in conveying the concepts of chemical bonding. It allowed me to explore the concept better.
- It allowed me to understand the concept better as it gave an explanation when providing the answer which was more comprehensive then just searching for the answer online.
- it was interactive and helped me to understand the lesson better
- I think it helps me understand where I went wrong and teaches me how to correct my mistakes before I write it down and have to go through the trouble of changing it over again and again.
- it was fun and easy to use!
- kept me engaged and aroused my interest
- it was successful because it was fun and interesting
In what way can the lesson or the simulation be improved? (For example, what features in the simulation would you like to change or add?)
- NIL
- Dragging and clicking of the electrons became tiring after a while, and it was very repetitive and could be more engaging. noted, We already designed a book icon that put all the electrons in appropriate positions, maybe need to tell students again?
- An option to include non-valence shells and a box of different colours of ions(we choose how many to take) but include the periodic table at the side.More effects and colours so it will be more attractive. If you can, thank you! noted, while the idea has merits, it may be too difficult when there is a unknown number electrons to position on the atomic shell, so it is unlikely i will implement that idea.
- It is a bit hard to drag the dots and crosses to their respective molecule. noted i have increase the sensitivity to 50px, meaning the dragging area is now 50x50px on the electrons O and X.
- the simulation can have more.
- -nil-
- We can only place the dots and crosses at certain places of the ring
- add a function that could help memorise the formulas or dot and cross diagrams better
- I think it is already very good.
- figure out how to make those who do not have touch screen laptop use more easily
- -
- it is slightly hard to drag the electrons and place them on their spots
- more graphics can be added to make the site more interesting.
- -
- Harder questions.
- Make the colours of the molecules in the simulation brighter as some were dull and
- i feel that the moving of dots was quite tiring and tedious
- The lesson can have more of these interactive games to keep us engaged.
- instead of drag and drop change it to click to grab and click to drop
- the simulation should have a function that allows us to pull all the electrons to the outer circle so we do not have to do it one by one.
- I would like there to be an explanation added in if the hint is used as seeing the answer alone does not help me understand the bond.
- make it easier to drop the electrons
- There can be some pop ups where the user can see what that compound is applied in daily lives so to spark more interest in learning these compounds:))
- it could be more aesthetically pleasing
- the toggle was a bit weird
- the electrons can be larger so it's easier to drag.
- NIL
- I think the thinking emoji can be changed to something like "Check" because some people didn't really know what to press to check the answer immediately. noted, i have added text check hint and reset.
- I find it tedious to move every single subatomic particle to its spot! I'd rather have them assigned to their respective atom and then move them around from there !
- make it more appealing including the colour lilac
- Instead of dragging the ‘x’ or the ‘o’ , we can tap them to move them from one place to another.
- there could be an explanation for why some ions are bonded that way
- nil
- - labels for buttons. done
- - better interface design
- Just select directly on the diagram instead of dragging it as it is quite tedious without touch screen.
- explanations should be given when students gets the answer incorrect.
- how to derive the answer
- Easier dragging of electrons cause sometimes it lags.
- More explanation (rgd concepts) please! Thank you! (esp for the last few qns)
- make it easier to drag and drop the electrons?
- There can be an explanation for the bonding after students submit each answer.
- would it be possible to have a wider range of compounds that we can try to do?
- it would be nice if there was an undo button rather than completely erasing what you've done, also its a bit tedious having to drag things over it may be nice if you can select an electron and place it in a spot
- pop up tutorial of how to use it. I wasn't really sure about how to check my answers. video coming
- more variety of compounds
- more graphics?
- The simulation could have a one step 'backspace' key instead of a total reset so we can undo step by step and do not have to reset when we want to backtrack.
- The simulation is really good and there is no improvements that I would want to see made.
- more covalent bonds/questions as the range of selection is small right now
- label the buttons as some students were confused on how to check the answer noted
- The simulation is a bit laggy.
- I think the explanations as to why our answers were wrong can be clearer as I was a bit confused when it first came out and didn't really understand what to do.
- the simulation could be more colourful
- automatically go on to the next qn
Any other feedback or comments for your teacher or the simulation designer? (This question allows students to feedback on any other matters. For example, you could thank your teacher or say how you feel about this simple technology enhanced lesson.)
- NIL
- -
- This app is really interesting. Thank you for spending time to make this app!
- it is fun
- -nil-
- I think the teacher should explain it more clearly first because I actually don't understand it before having to explore the simulation. I feel appreciative that the simulation designer designed this thing that helped me to understand more about the topic.
- quite great
- -
- Using technology during lessons is more fun. :)
- :D
- The simulation was really cool! Thank you so much!
- Thank you Ms T for giving us time to try out this simulation and allowing us to learn on our own! :)
- The simulation is much faster and more efficient than physically drawing covalent bonds.
- Thank you for the lesson!
- nil
- This simulation is a really great way to get students to practise and they can see how to draw and where to put the electrons in the correct places!!
- it is quite hard to drag the electron , noted sensitivity set to 50 px
- thank you for the lesson
- thanks haha:))
- NIL
- Thank you Ms T for trusting us to do our own self- learning ! Despite you not actively teaching us, it was a simple but fruitful, hands-on lesson ^-^
- thank you v much heehee
- -
- -
- thank you for the website
- THANk You MS C T
- i enjoyed the lesson using the simulation very much! thank you!
- thanks
- Thank you for letting us use this simulation.
- It's really good :)
- Thank you for creating this!
- Thank you for spending so much time and effort to create this simulation make our lessons more interesting!
- This lesson was very fun! Thank you for making the simulation!!
- thank you for this simulation. it was simple but effective
- thank you for making this programme !
- Would it be possible to have the electrons of the atoms to be an option to click on before filling in the configuration,so we don't have to drag the ones given to their rightful place?
- I like this.
- N/A
- it was really fun!!!
Video
Version:
- https://weelookang.blogspot.com/2019/12/chemical-bonding-dot-and-cross-diagrams.html
- https://sites.google.com/a/mgs.sch.edu.sg/about-me-david-loh/
1. Dot and Cross Diagram 8
https://sg.iwant2study.org/ospsg/index.php/922-dotandcrossdiagram82. Dot and Cross Diagram 7 A-Level
https://sg.iwant2study.org/ospsg/index.php/923-dotandcrossdiagram7alevel3. Dot and Cross Diagram 8 with Valency Option
https://sg.iwant2study.org/ospsg/index.php/1006-dotandcrossdiagram8withvalencyoption4. Dot and Cross Diagram 8 – Methane
https://sg.iwant2study.org/ospsg/index.php/1111-dotandcrossdiagram8-methane5. Dot and Cross Diagram 8 – Ammonia
https://sg.iwant2study.org/ospsg/index.php/1112-dotandcrossdiagram8-ammonia6. Chemistry Interactive Resources (All)
https://sg.iwant2study.org/ospsg/index.php/interactive-resources/chemistry
Other Resources
[text]
Frequently Asked Questions: Covalent Bonding 'Dot and Cross' Diagrams Simulation
1. What is the purpose of the Covalent Bonding 'Dot and Cross' Diagrams Simulation?
The primary purpose of the simulation is to help students learn and visualize the formation of covalent bonds in various molecules and polyatomic ions. It provides an interactive platform where users can represent valence electrons as dots and crosses to understand how atoms share electrons to achieve a stable electron configuration, typically resembling a noble gas (octet rule, or duet rule for hydrogen).
2. Who created this simulation and under what licensing is it released?
This simulation was written by Loo Kang Wee, Felix J. Garcia Clemente, and Francisco Esquembre, and designed by David Loh. It is © 2023 and released under a Creative Commons Attribution-Share Alike-Non-Commercial (CC-BY-SA-NC) license. The software used to compile it is EJS 6.1 BETA (201115).
3. What are the key learning goals associated with using this simulation?
The main learning goals include understanding how covalent bonds are formed through the sharing of electrons, being able to draw 'dot and cross' diagrams for various molecules and polyatomic ions, and visualizing how atoms achieve a stable outer electron shell configuration (octet or duet) through covalent bonding.
4. Which chemical species can be explored using this simulation?
The simulation offers examples of 'dot and cross' diagrams for a variety of molecules and polyatomic ions, including Hydrogen (H₂), Chlorine (Cl₂), Oxygen (O₂), Nitrogen (N₂), Hydrogen Chloride (HCl), Hydroxide ion (OH⁻), Cyanide ion (CN⁻), Water (H₂O), Carbon Dioxide (CO₂), Nitrite ion (NO₂⁻), Ammonia (NH₃), Methane (CH₄), Carbonate ion (CO₃²⁻), and Hydrogen Peroxide (H₂O₂).
5. How does the simulation help students learn about covalent bonding?
The simulation offers a hands-on and interactive approach to learning. Students can manipulate electrons (represented as dots and crosses) and place them around the atomic symbols to form covalent bonds. The simulation often provides feedback or model answers, allowing students to check their understanding and learn from their mistakes. Many students found it more engaging and easier to visualize compared to drawing diagrams on paper.
6. What features of the simulation are particularly helpful for learning?
Students have highlighted several helpful features, including the ability to visualize the concepts, the interactive nature of dragging and dropping electrons, the provision of the number of valence electrons, the availability of hints and model answers, and the immediate feedback on their attempts. The color-coding of electrons from different atoms also aids in understanding the sharing process.
7. What improvements or additional features have students suggested for the simulation?
Students have suggested several improvements, such as making the dragging of electrons less tiring, adding more graphics and aesthetic appeal, including explanations for incorrect answers or the bonding process, providing an undo button, offering a wider range of compounds to explore, and improving the interface for non-touchscreen devices (e.g., click-to-grab and click-to-drop functionality). Some also requested real-world applications of the compounds to increase interest.
8. Has the effectiveness of this simulation been evaluated, and what were the findings?
Surveys conducted with students in Singapore schools suggest strong evidence of effective student learning, appreciative students, and good simulation design. Students reported that the simulation helped them understand the concepts better, made learning more interesting and engaging, and allowed for independent learning and practice with immediate feedback.
- Details
- Written by David Loh
- Parent Category: Chemistry
- Category: 03 Chemistry of Reactions
- Hits: 32028


.png
)