Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits



 Written by Loo Kang Wee; Felix J. Garcia Clemente; Francisco Esquembre; Designed by David Loh
Written by Loo Kang Wee; Felix J. Garcia Clemente; Francisco Esquembre; Designed by David Loh
This briefing document reviews two sources detailing interactive JavaScript simulations designed to teach the concept of covalent bonding, with a specific focus on the methane molecule. The simulations utilize the "dot and cross" diagram method to visually represent the sharing of electrons between atoms.
Source 1: "Covalent Bonding 'Dot and Cross' Simulation only methane"
- Authors/Developers: Loo Kang Wee, Felix J. Garcia Clemente, Francisco Esquembre; Designed by David Loh
- License: CC-BY-SA-NC (Creative Commons Attribution-ShareAlike-NonCommercial)
- Key Theme: This source directly focuses on a simulation specifically designed for the methane molecule. The title indicates its singular focus, suggesting a targeted tool for understanding the bonding in CH₄.
- Important Ideas/Facts:The simulation utilizes the "dot and cross" method, a common visual representation in introductory chemistry for illustrating covalent bonds.
- The mention of "Compiled with EJS 6.1 BETA (201115)" indicates the software used to create the interactive element, likely Easy JavaScript Simulations (EJS), an open-source tool for creating science and mathematics simulations.
- The CC-BY-SA-NC license signifies that the resource can be shared and adapted for non-commercial purposes, provided attribution is given and any adaptations are shared under a similar license.
Source 2: "O level Chemical Methane Covalent Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5 - Open Educational Resources / Open Source Physics @ Singapore"
- Source: Open Educational Resources / Open Source Physics @ Singapore
- Authors/Developers: Loo Kang Wee, Felix J. Garcia Clemente, Francisco Esquembre; Designed by David Loh (same as Source 1)
- Key Themes: This source provides a broader context, positioning the methane covalent bonding simulation within a larger collection of open educational resources for physics and chemistry in Singapore. It highlights the use of interactive simulations as a pedagogical tool and includes feedback from students who have used similar simulations.
- Important Ideas/Facts:Methane as a Specific Example: The resource explicitly mentions "O level Chemical Methane Covalent Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5," confirming the availability of a methane-specific simulation within this collection.
- Embeddable Resource: The provision of an <iframe> code allows educators to easily embed the simulation into their own webpages or learning management systems.
- Credits and Development: The authors and designers are credited, reinforcing the collaborative nature of the resource development.
- Learning Goals: The source lists "O level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5" and "A Level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5" as sample learning goals, indicating the target audience and educational level.
- Teacher Support: The resource encourages teachers in Singapore schools to contact a specific email address to obtain a digital Google survey form for collecting data on the effectiveness of the lesson and providing feedback for future improvements. This highlights a commitment to iterative development based on real-world classroom use.
- Evidence of Effectiveness: The website states, "Our own survey suggests strong evidence on effective student learning, appreciative students, good simulation design." This claim is further supported by a link to a blog post with more details and a collection of student feedback.
- Broader Range of Molecules and Ions: While the briefing focuses on methane, this source also lists direct links to "Chemical Bonding Dot and Cross Diagrams" for various other molecules (Hydrogen, Chlorine, Oxygen, Nitrogen, Hydrogen Chloride, Water, Carbon Dioxide, Ammonia, Hydrogen Peroxide) and polyatomic ions (Hydroxide, Cyanide, Nitrite, Carbonate). This suggests a wider suite of simulations available.
- Student Feedback: A significant portion of the source includes direct student feedback on lessons using similar interactive dot and cross diagram simulations. Key takeaways from this feedback include:
- Engagement and Interest: Students found the simulations "interesting," "interactive," and a "more interesting style of learning" compared to traditional methods like drawing on paper or using Google Slides. Quotes include: "The simulation made the lesson more interesting as it was interactive," and "It keeps my interest because it is easy to use."
- Improved Understanding and Visualization: Many students reported that the simulations helped them "visualise the concepts" and understand chemical bonding better. Examples: "It was helpful to visualise the concepts," and "The simulation helps me to understand better about chemical bonding dot & cross diagram."
- Ease of Use and Accessibility: Students appreciated the ease of use, the ability to learn independently, and the immediate feedback provided. Quotes include: "the simulation was easy to use and easy to understand," and "It allowed me to understand more about the formation of covalent bonds."
- Advantages over Traditional Methods: Students noted the efficiency and convenience compared to drawing manually. "The simulation is much easier than drawing the atoms out," and "It was a lot easier to correct small errors using the simulation that it is with pen and paper."
- Desire for Improvements: Students also offered suggestions for improvement, such as making dragging electrons easier, adding more compounds, providing explanations for incorrect answers, and including an undo button. One student noted the tediousness of dragging: "dragging and clicking of the electrons became tiring after a while." Another suggested: "instead of drag and drop change it to click to grab and click to drop."
- Teacher Integration: The inclusion of Google Slides for teachers indicates an effort to provide supplementary materials for educators using the simulations.
- Emphasis on Trial and Error: Some student feedback highlights the value of being able to "trial and error" and "figure out the bonding by myself" using the simulation.
Quotes Highlighting Key Ideas:
- Simulation Design (Implicit): The focus on "Dot and Cross Diagrams" in both titles indicates the core visual representation used in the simulations.
- Evidence of Effectiveness: "Our own survey suggests strong evidence on effective student learning, appreciative students, good simulation design."
- Student Engagement: "The simulation made the lesson more interesting as it was interactive." (Student Feedback)
- Improved Understanding: "The simulation helps me to understand better about chemical bonding dot & cross diagram." (Student Feedback)
- Ease of Use: "The simulation was easy to use and easy to understand." (Student Feedback)
- Advantage over Traditional Methods: "The simulation is much easier than drawing the atoms out." (Student Feedback)
- Suggestion for Improvement: "Dragging and clicking of the electrons became tiring after a while..." (Student Feedback)
Conclusion:
Both sources highlight the development and use of interactive JavaScript simulations based on "dot and cross" diagrams as a valuable tool for teaching covalent bonding, specifically using methane as an example. The resources are openly licensed, embeddable, and have been used in educational settings, garnering positive feedback from students regarding engagement, understanding, and ease of use. The feedback also provides valuable insights for potential improvements to the simulations. The broader collection of simulations available through the "Open Educational Resources / Open Source Physics @ Singapore" platform suggests a comprehensive resource for teaching chemical bonding concepts at different levels. Educators looking for engaging and effective ways to teach covalent bonding could significantly benefit from exploring and utilizing these simulations.
Covalent Bonding of Methane: A Study Guide
Key Concepts
- Covalent Bond: A chemical bond that involves the sharing of electron pairs between atoms.
- Molecule: A group of two or more atoms held together by chemical bonds.
- Methane (CH₄): A molecule consisting of one carbon atom and four hydrogen atoms. It is the simplest alkane.
- Valence Electrons: The electrons in the outermost shell of an atom that are involved in chemical bonding.
- Dot and Cross Diagram: A visual representation of covalent bonding showing the valence electrons of different atoms as dots and crosses, illustrating how they are shared.
- Electron Shell: The regions around the nucleus of an atom where electrons are likely to be found at a particular energy level.
- Octet Rule (and Duet Rule): The tendency of atoms to prefer to have eight electrons in their valence shell (or two electrons in the case of hydrogen) to achieve stability.
- Sharing of Electrons: The fundamental process in covalent bonding where atoms contribute one or more electrons to be mutually shared, resulting in a stable electron configuration for each atom.
- Stability: The state of an atom or molecule with a complete valence electron shell, making it less reactive.
Quiz
- Describe the formation of a covalent bond in methane (CH₄). In your explanation, mention the atoms involved and the electrons that participate in bonding.
- What is the purpose of using dots and crosses in a bonding diagram? How does this notation help visualize the formation of covalent bonds in methane?
- How many valence electrons does a carbon atom have, and how many does a hydrogen atom have? Explain how this relates to the bonding in methane.
- Explain how the concept of electron shells is represented in a dot and cross diagram of methane. What does the diagram show about the electron configuration of carbon and hydrogen after bonding?
- What is the significance of each hydrogen atom in methane achieving a "duet" and the carbon atom achieving an "octet" of electrons? How does this relate to the stability of the methane molecule?
- In the dot and cross diagram of methane, how many electron pairs are shared? What does each shared pair of electrons represent in terms of chemical bonding?
- According to the student feedback provided, what are some perceived benefits of using a simulation for learning about covalent bonding in methane? Provide at least two distinct advantages mentioned by students.
- Based on the student feedback, identify at least two suggestions for improving the covalent bonding simulation.
- What does the license information (CC-BY-SA-NC) associated with the "Covalent Bonding 'Dot and Cross' Simulation only methane" resource indicate about its use and distribution?
- Explain why methane is considered a molecule. What distinguishes it from individual carbon and hydrogen atoms?
Quiz Answer Key
- A covalent bond in methane forms when one carbon atom shares its four valence electrons with four hydrogen atoms. Each hydrogen atom contributes one valence electron, resulting in four shared pairs of electrons that hold the atoms together.
- Dots and crosses are used to distinguish the valence electrons originating from different atoms in a covalent bond. This visual notation clearly shows how electrons are shared between carbon (typically represented by crosses) and hydrogen (typically represented by dots) to form the bonds in methane.
- A carbon atom has four valence electrons, while a hydrogen atom has one valence electron. In methane, the carbon atom needs four more electrons to achieve an octet, and each of the four hydrogen atoms needs one more electron to achieve a duet, hence the sharing of electrons in four covalent bonds.
- Electron shells are implicitly represented by showing the valence electrons around the atomic symbols. The dot and cross diagram for methane shows carbon surrounded by eight electrons (its own four and one from each of the four hydrogens), and each hydrogen surrounded by two electrons (its own one and one from the carbon), indicating filled outer shells.
- Achieving a duet (for hydrogen with two electrons) and an octet (for carbon with eight electrons in its valence shell through sharing) results in a more stable electron configuration similar to noble gases. This increased stability is the driving force behind the formation of covalent bonds in methane.
- In the dot and cross diagram of methane, there are four shared pairs of electrons, with each pair consisting of one electron from carbon and one electron from a hydrogen atom. Each shared pair represents a single covalent bond between the carbon and one hydrogen atom.
- Students found the simulation helpful for visualizing the concepts of covalent bonding and for providing a more hands-on and interactive learning experience compared to traditional methods. They also appreciated the immediate feedback and the ability to learn independently.
- Some suggestions for improvement included making the dragging and dropping of electrons easier, adding more visual appeal (like more graphics or brighter colors), and potentially including explanations for incorrect answers or the reasons behind the bonding.
- The CC-BY-SA-NC license indicates that the resource can be shared and adapted (even commercially) as long as attribution is given to the creators, the resulting work is shared under the same or a similar license, and it is not used for commercial purposes.
- Methane is considered a molecule because it is formed when one carbon atom and four hydrogen atoms are chemically bonded together through the sharing of electrons (covalent bonds). These bonds create a stable and distinct entity with properties different from the individual atoms.
Essay Format Questions
- Discuss the significance of dot and cross diagrams in understanding the formation of covalent bonds, using methane as a specific example. How do these diagrams help to illustrate the octet and duet rules in the context of molecular stability?
- Analyze the feedback provided by students on the use of the covalent bonding simulation for methane. What are the key strengths and weaknesses of this learning tool, and how could such simulations be further optimized for chemistry education?
- Explain the process of covalent bond formation in methane from the perspective of electron sharing and the achievement of stable electron configurations. Detail the role of valence electrons in this process.
- Compare and contrast the representation of chemical bonding using dot and cross diagrams with other methods you might know (e.g., Lewis structures, structural formulas). What are the advantages and limitations of each approach in understanding the structure and bonding in methane?
- Based on the provided resources, discuss the potential benefits of using interactive simulations, like the one for methane's covalent bonding, in enhancing student engagement and understanding in chemistry education. Support your arguments with evidence from the student feedback.
Glossary of Key Terms
- Atom: The basic unit of a chemical element.
- Chemical Bond: An attraction between atoms or ions that enables the formation of chemical compounds.
- Compound: A substance formed when two or more chemical elements are chemically bonded together.
- Electron: A stable subatomic particle with a negative electric charge.
- Lewis Structure: A diagram that shows the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- Nucleus: The small, dense region at the center of an atom, consisting of protons and neutrons.
- Proton: A stable subatomic particle with a positive electric charge, found in the nucleus of an atom.
- Structural Formula: A representation of the molecular structure in which the bonding electron pairs are shown either explicitly as bonds or implicitly by the spatial arrangement of atoms.
- Valency: The combining capacity of an atom or ion; the number of chemical bonds that an atom of a given element can form.
https://play.google.com/store/apps/details?id=com.ionicframework.covalentbonding this version has the valency question
Sample Learning Goals
- A Level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5
- O level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5
For Teachers
From Google slides (From David) to interactive https://docs.google.com/presentation/d/1fwutLc-jPc1fUyrxJsps3Fhg6_J9y8AEA4pHJ68gdBw/edit?ts=5dd2086a#slide=id.g5292a6c619_0_96
If you are using this simulation in Singapore Schools, please contact This email address is being protected from spambots. You need JavaScript enabled to view it. to get a digital google survey form to collect anonymous data on the effectiveness of lesson and future imporvement to the simulation!. Thanks in advance.
Our own survey suggests strong evidence on effective student learning, appreciative students, good simulation design. Do you agree? let me know in the comments below! https://weelookang.blogspot.com/2020/02/o-level-chemical-bonding-dot-and-cross.html
Chemical Bonding Dot and Cross Diagrams
Polyatomic ions dot and cross diagram
|
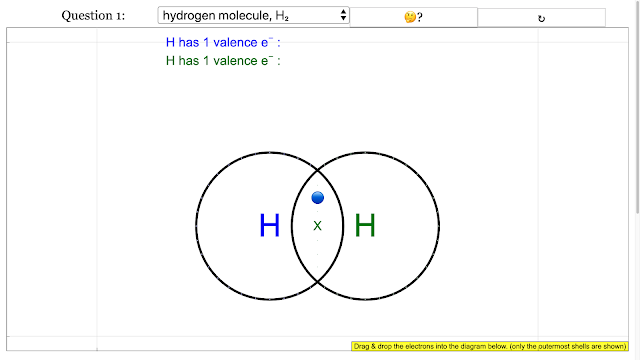
Chemical Bonding Dot and Cross Diagrams for Hydrogen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen molecule answer is add up to 2 on each H atom electron outermost with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Chlorine molecule |
|
Chemical Bonding Dot and Cross Diagrams for Chlorine molecule answer is add up to 8 on each atom's' electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Oxygen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Oxygen molecule answer is is add up to 8 on each atom's' electron outermost shell with shared electrons = 4 |
|
Chemical Bonding Dot and Cross Diagrams for Nitrogen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Nitrogen molecule answer is is add up to 8 on each atom's' electron outermost shell with shared electrons = 6 |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Chloride molecule |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Chloride molecule answer is is add up to 8 on Cl atom and 2 for H atom and electron outermost shell with shared electrons = 2 |
|
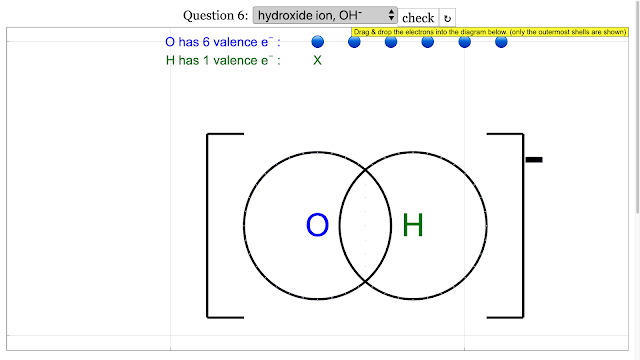
Chemical Bonding Dot and Cross Diagrams for Hydroxide ion |
|
Chemical Bonding Dot and Cross Diagrams for Hydroxide ion answer is is add up to 8 on O atom and 2 for H atom and electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Cyanide ion |
|
Chemical Bonding Dot and Cross Diagrams for Cyanide ion answer is is add up to 8 on each atoms and electron outermost shell with shared electrons = 6 |
|
Chemical Bonding Dot and Cross Diagrams for Water molecule |
|
Chemical Bonding Dot and Cross Diagrams for Water molecule answer is is add up to 8 on O atom and 2 on H atoms and electron outermost shell with shared electrons = 2,2 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Carbon Dioxide molecule |
|
Chemical Bonding Dot and Cross Diagrams for Carbon Dioxide molecule answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 4,4 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 2,4 respectively with the foreign electron on the O atom with the shared electrons=2 |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 2,4 respectively with the foreign electron on the O atom with the shared electrons=2 |
|
Chemical Bonding Dot and Cross Diagrams for Ammonia molecule |
|
Chemical Bonding Dot and Cross Diagrams for Ammonia molecule answer is is add up to 8 on N atom and 2 on H atom and the electron outermost shell with shared electrons = 2,2,2 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Methane molecule |
|
Chemical Bonding Dot and Cross Diagrams for Methane molecule answer is is add up to 8 on C atom and 2 on H atom and the electron outermost shell with shared electrons = 2,2,2, respectively |
|
Chemical Bonding Dot and Cross Diagrams for Carbonate ion |
|
Chemical Bonding Dot and Cross Diagrams for Carbonate ion answer is is add up to 8 on each atom and the electron outermost shell with shared electrons = 4,2,2, respectively. The two O atoms with 2 shared electrons has a foreign electron in it's personal shell. |
 |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Peroxide |
Student Survey
survey 1 , 2 data from MGS 2020 total 1 + 2 classes estimated number of students (44 , 46) responses.
I have added some answers to the questions asked too.
 |
| typical classroom setting where the anonymous (photo blurred) teacher skillfully explains the learning tasks using https://sg.iwant2study.org/ospsg/index.php/922 |
 |
| typical classroom setting where the anonymous students are each working on their ChromeBook to learn by doing chemistry. this is far better than students working on Google Slides and trying to fit in the electrons on non simulation medium, only getting feedback on the accuracy after the teacher manually marked each students' assigned google slide prepared by the teacher. https://sg.iwant2study.org/ospsg/index.php/922 |
What do you like about the lesson? (For example, was the lesson successful in helping you to learn the concepts? In what way was the simulation helpful? Did it arouse your interest? Please give more details.)
- It was successful in helping me learn the basic concepts but it did not give me a very clear understanding of the concept. I was still left with questions and doubts. It was a unique and new way of learning in the classroom. noted, Video Tutorial will be ready after march holidays.
- Something new. No need to check the periodic table. There are other compounds not tested available. The emoji is cute.
- It was helpful to visualise the concepts.
- the lesson was successful in helping me to learn the concepts. the simulation was helpful. It arouse my interest.
- it is interesting and can help me learn independently.
- The lesson was more hands on than just the teacher explaining to us how to draw dot and cross diagrams
- This is a more interesting style of learning
- The simulation made the lesson more interesting as it was interactive
- the simulation was easy to use and easy to understand
- The simulation helps me to understand better about chemical bonding dot & cross diagram. I also can engage with the lesson better because we get to use technology during lessons and there are colours.
- yes,it helps me a lot ,I was confused about the definition yesterday night ,but through this lesson, I think I know better about the definition and the process .
- it helped me to understand better
- the simulation made me more interested in learning about chemical bonding.
- I like the fact that we got to learn through using our chromebook and got to try it out ourselves.
- it allowed me to understand more about the formation of covalent bonds
- I was fun and was an easier and more interesting way to learn about covalent bonding instead of literally drawing the atom over and over again.
- It keeps my interest because it is easy to use
- it was interesting i guess
- It was interesting and helped me to be more confident in dot and cross diagrams.
- that we get to attempt it ourselves first
- It is easy to use and informative
- it was easy to understand with answers and hints provided
- The simulation was cute and captured my attention so I enjoyed doing it.
- The simulation allowed me to discover where the electrons should go and somehow, why it was like that.
- it's easier to use
- it was easy and fun to use
- The lesson was successful in helping me learn the concepts. It gave me more practice in covalent bonding.
- the simulation gave me a concept of what covalent bonding is about
- The simulation is much easier than drawing the atoms out.
- I like that the lesson allowed me to learn independently and the diagrams were simple and easy to read.
- The lesson helped me have a clear visual on covalent bonding. Helped it to be clearer to me.
- The answers are not given on the first wrong attempt, we can continue to try to get to the correct answer.
- The simulation made the locations of the electrons clearer
- I like that it gives the number of electrons that the user has to use and that the user does not need to refer to the periodic table to do this simulation, increasing interest and ease in doing this while learning something.
- It was a refreshing change. Instead of drawing the diagrams on paper, I could move the electrons online.
- it is very entertaining and educative
- the visuals helped me!
- it was interesting
- The colour coding makes the visuals much more easier to see and understand the concepts.
- The lesson was very fun and engaging. The simulation allowed me to better understand the concepts.
- It was successful in helping me learn the concepts as it provided a solution and explanation when i didn't know how to do the question.There were also many question which allowed me to try out different questions or different levels of difficulty,allowing me to practice more.
- it was fun
- The hints especially helped me to understand the concept
- I can understand the structures more clearly. was quite fun to drag and drop instead of drawing for once. It's also much more convenient as i can check my work immediately instead of waiting for the teacher to come around
- The simulation was easy to use and was a good reference for doing the worksheet as well !
- the simulation was helpful in giving model answers for me to understand covalent bonding better
- The simulation helped me determine the number of valence electrons so it was easier to draw the dot and cross diagram. The simulation was very fun and easy to use and it was a drag and drop application
- was very successful
- the simulation helped me better understand the topic better and it was engaging
- I liked that it was more engaging and gave us room to think for ourselves while having some guidance from the simulation. The simulation made it easier to trial and error so that I could figure out the bonding by myself.
- it is interactive and clear:)
- It helped me understand the concept of bonding better. It was very interesting and helpful. I would like to do it again in class sometime.
- the lesson was easy to follow since the simulation was easy to use and we could just reset instead of redrawing
- It was a lot easier to correct small errors using the simulation that it is with pen and paper.
- It was pretty interactive and it helped us to apply important concepts when drawing dot-cross diagrams.
- - easy to use
- - I had more confidence in drawing dot and cross diagrams
- - wide range of ions to choose from
- - "hints" feature
- It was easy to use and a suitable amount of help was given (number of valence electrons per element were given)
- i liked how it was not too complicated to do the dot-and-cross diagrams and the website was easy to use.
- helpful - it prompted us and gave hints on how to draw the diagram
- yes i am interested
- it was good to check whether my diagrams were right
- Yes it was very helpful. I learnt how to draw polyatomic ions very quickly and I find this kind of simulation useful as I can self-learn without relying so much on the teacher. :)
- It gives a better visualisation of the dot and cross diagram and helps me to be able to draw the diagram easily.
- It made the process of thinking how to draw it much easier
- i was able to learn the concept faster with the use of the website
- It helped me visualise the covalent bonding between the molecules and made me curious to find out why the molecules bonded in a particular way
- I liked using the simulation to trial and error with the diagram and to fill in the worksheet.
- the platform made learning the dot cross diagram more interesting
- it helped me figure out the answer in my own.
- the lesson helped to make my understanding of covalent bonding clearer
- I liked the idea of figuring it out yourself and then having a check answer button this app is great!
- It made visualising the covalent bonds and the orientation of electrons easier.
- ー
- it is fun to drag the electrons around
- it gives me many chances for trial and error without having to continuously erase my mistakes
- I like that we get to explore the concept ourselves and learn by trial and error.
- The simulation was easy to use and allowed me to understand how to draw dot and cross diagrams as it told me what was wrong when I made a mistake
- I learnt how foreign electrons worked, and it was interesting to see try doing the different questions. It was also easier as the simulation already had the electrons shells put out for you.
- It helped me to visualise the concept better
- I liked the lesson because it was more engaging and hands-on, and it also helped me to learn the concepts. It is also better than having to draw out a dot and cross diagram myself.
- It helped me to see the chemical bonds visually which was very helpful
- It allowed me to easily move each electron around before writing it on my paper thus it was easier and neater. Furthermore, because of the difference colours it is easier to see the electrons
- The simulation made it easier for me to visualise the atoms, aiding me in figuring out the configuration of different compounds.
- It was successful in conveying the concepts of chemical bonding. It allowed me to explore the concept better.
- It allowed me to understand the concept better as it gave an explanation when providing the answer which was more comprehensive then just searching for the answer online.
- it was interactive and helped me to understand the lesson better
- I think it helps me understand where I went wrong and teaches me how to correct my mistakes before I write it down and have to go through the trouble of changing it over again and again.
- it was fun and easy to use!
- kept me engaged and aroused my interest
- it was successful because it was fun and interesting
In what way can the lesson or the simulation be improved? (For example, what features in the simulation would you like to change or add?)
- NIL
- Dragging and clicking of the electrons became tiring after a while, and it was very repetitive and could be more engaging. noted, We already designed a book icon that put all the electrons in appropriate positions, maybe need to tell students again?
- An option to include non-valence shells and a box of different colours of ions(we choose how many to take) but include the periodic table at the side.More effects and colours so it will be more attractive. If you can, thank you! noted, while the idea has merits, it may be too difficult when there is a unknown number electrons to position on the atomic shell, so it is unlikely i will implement that idea.
- It is a bit hard to drag the dots and crosses to their respective molecule. noted i have increase the sensitivity to 50px, meaning the dragging area is now 50x50px on the electrons O and X.
- the simulation can have more.
- -nil-
- We can only place the dots and crosses at certain places of the ring
- add a function that could help memorise the formulas or dot and cross diagrams better
- I think it is already very good.
- figure out how to make those who do not have touch screen laptop use more easily
- -
- it is slightly hard to drag the electrons and place them on their spots
- more graphics can be added to make the site more interesting.
- -
- Harder questions.
- Make the colours of the molecules in the simulation brighter as some were dull and
- i feel that the moving of dots was quite tiring and tedious
- The lesson can have more of these interactive games to keep us engaged.
- instead of drag and drop change it to click to grab and click to drop
- the simulation should have a function that allows us to pull all the electrons to the outer circle so we do not have to do it one by one.
- I would like there to be an explanation added in if the hint is used as seeing the answer alone does not help me understand the bond.
- make it easier to drop the electrons
- There can be some pop ups where the user can see what that compound is applied in daily lives so to spark more interest in learning these compounds:))
- it could be more aesthetically pleasing
- the toggle was a bit weird
- the electrons can be larger so it's easier to drag.
- NIL
- I think the thinking emoji can be changed to something like "Check" because some people didn't really know what to press to check the answer immediately. noted, i have added text check hint and reset.
- I find it tedious to move every single subatomic particle to its spot! I'd rather have them assigned to their respective atom and then move them around from there !
- make it more appealing including the colour lilac
- Instead of dragging the ‘x’ or the ‘o’ , we can tap them to move them from one place to another.
- there could be an explanation for why some ions are bonded that way
- nil
- - labels for buttons. done
- - better interface design
- Just select directly on the diagram instead of dragging it as it is quite tedious without touch screen.
- explanations should be given when students gets the answer incorrect.
- how to derive the answer
- Easier dragging of electrons cause sometimes it lags.
- More explanation (rgd concepts) please! Thank you! (esp for the last few qns)
- make it easier to drag and drop the electrons?
- There can be an explanation for the bonding after students submit each answer.
- would it be possible to have a wider range of compounds that we can try to do?
- it would be nice if there was an undo button rather than completely erasing what you've done, also its a bit tedious having to drag things over it may be nice if you can select an electron and place it in a spot
- pop up tutorial of how to use it. I wasn't really sure about how to check my answers. video coming
- more variety of compounds
- more graphics?
- The simulation could have a one step 'backspace' key instead of a total reset so we can undo step by step and do not have to reset when we want to backtrack.
- The simulation is really good and there is no improvements that I would want to see made.
- more covalent bonds/questions as the range of selection is small right now
- label the buttons as some students were confused on how to check the answer noted
- The simulation is a bit laggy.
- I think the explanations as to why our answers were wrong can be clearer as I was a bit confused when it first came out and didn't really understand what to do.
- the simulation could be more colourful
- automatically go on to the next qn
Any other feedback or comments for your teacher or the simulation designer? (This question allows students to feedback on any other matters. For example, you could thank your teacher or say how you feel about this simple technology enhanced lesson.)
- NIL
- -
- This app is really interesting. Thank you for spending time to make this app!
- it is fun
- -nil-
- I think the teacher should explain it more clearly first because I actually don't understand it before having to explore the simulation. I feel appreciative that the simulation designer designed this thing that helped me to understand more about the topic.
- quite great
- -
- Using technology during lessons is more fun. :)
- :D
- The simulation was really cool! Thank you so much!
- Thank you Ms T for giving us time to try out this simulation and allowing us to learn on our own! :)
- The simulation is much faster and more efficient than physically drawing covalent bonds.
- Thank you for the lesson!
- nil
- This simulation is a really great way to get students to practise and they can see how to draw and where to put the electrons in the correct places!!
- it is quite hard to drag the electron , noted sensitivity set to 50 px
- thank you for the lesson
- thanks haha:))
- NIL
- Thank you Ms T for trusting us to do our own self- learning ! Despite you not actively teaching us, it was a simple but fruitful, hands-on lesson ^-^
- thank you v much heehee
- -
- -
- thank you for the website
- THANk You MS C T
- i enjoyed the lesson using the simulation very much! thank you!
- thanks
- Thank you for letting us use this simulation.
- It's really good :)
- Thank you for creating this!
- Thank you for spending so much time and effort to create this simulation make our lessons more interesting!
- This lesson was very fun! Thank you for making the simulation!!
- thank you for this simulation. it was simple but effective
- thank you for making this programme !
- Would it be possible to have the electrons of the atoms to be an option to click on before filling in the configuration,so we don't have to drag the ones given to their rightful place?
- I like this.
- N/A
- it was really fun!!!
Video
Version:
- https://weelookang.blogspot.com/2019/12/chemical-bonding-dot-and-cross-diagrams.html
- https://sites.google.com/a/mgs.sch.edu.sg/about-me-david-loh/
- https://weelookang.blogspot.com/2021/09/o-level-chemical-ammonia-and-methane.html
Other Resources
[text]
Frequently Asked Questions: Covalent Bonding and Methane Simulation
- What is covalent bonding, and how does the methane simulation illustrate it? Covalent bonding is a type of chemical bond where atoms share one or more pairs of electrons to achieve a stable electron configuration, typically resembling a noble gas with a full outer shell. The methane simulation specifically focuses on this type of bonding in the methane (CH₄) molecule. It allows users to visualize how a carbon atom (with 4 valence electrons) shares one electron with each of the four hydrogen atoms (each with 1 valence electron). This sharing results in carbon having a total of 8 electrons in its outer shell (a stable octet) and each hydrogen atom having 2 electrons in its outer shell (a stable duet, like helium). The "dot and cross" diagrams in the simulation visually represent the valence electrons of each atom and how they are shared in the covalent bonds.
- What is a "dot and cross" diagram, and how is it used in the methane simulation? A "dot and cross" diagram is a visual representation of covalent bonding. Dots and crosses are used to represent the valence electrons (outermost shell electrons) of different atoms involved in the bond. In the methane simulation, you would typically see a central carbon atom with either dots or crosses representing its 4 valence electrons, surrounded by four hydrogen atoms, each with the opposite symbol representing its 1 valence electron. The overlapping regions or shared space between the carbon and hydrogen atoms contain a pair of one dot and one cross, indicating that one electron from each atom is being shared, forming a covalent bond. The simulation allows users to manipulate these electrons to correctly form the methane molecule's dot and cross diagram, reinforcing the concept of electron sharing.
- Why is methane used as the sole example in this specific simulation? Methane (CH₄) is a relatively simple molecule with a clear and fundamental example of covalent bonding. It involves one central atom (carbon) bonded to four identical atoms (hydrogen) through single covalent bonds. This simplicity makes it an ideal starting point for understanding the basic principles of covalent bonding and how dot and cross diagrams are constructed. By focusing on a single, straightforward example, the simulation can effectively teach the core concepts without the complexities of multiple bonds or different types of covalent bonds found in larger molecules.
- Who created this methane covalent bonding simulation, and what is its purpose? The methane covalent bonding simulation was written by Loo Kang Wee, Felix J. Garcia Clemente, and Francisco Esquembre, and designed by David Loh. It is part of the Open Educational Resources / Open Source Physics @ Singapore project. Its primary purpose is to provide an interactive and visual tool for students (particularly O level and A level chemistry students) to learn about covalent bonding through the specific example of methane. The simulation aims to make the abstract concept of electron sharing more concrete and engaging, offering a hands-on approach to understanding how molecules like methane are formed.
- How can teachers use this simulation in their lessons on chemical bonding? Teachers can integrate this simulation into their lessons in various ways. It can be used as an introductory tool to visually demonstrate covalent bond formation in methane. Students can use it individually on their devices (like Chromebooks) as a self-learning activity, allowing them to experiment with electron arrangements and receive immediate feedback. Teachers can also use it for whole-class demonstrations, perhaps projected onto a screen, to explain the concepts. The simulation offers a more interactive alternative to traditional methods like drawing diagrams on paper or using static slides. The feedback from students suggests it increases engagement and helps visualize abstract concepts. Teachers are also encouraged to use a provided digital Google survey to collect anonymous data on the simulation's effectiveness for future improvements.
- What are some of the reported benefits of using this type of interactive simulation for learning chemical bonding? Student feedback collected on the use of this and similar simulations indicates several benefits. These include: improved visualization of abstract concepts, increased engagement and interest in the topic, the opportunity for independent learning and self-correction through trial and error, immediate feedback on their understanding, and a more hands-on learning experience compared to traditional methods. Students also appreciate not needing to constantly refer to the periodic table as the number of valence electrons is often provided within the simulation. The interactive nature of dragging and dropping electrons is seen as more engaging than simply drawing diagrams.
- What are some of the suggestions for improving the methane covalent bonding simulation based on user feedback? User feedback suggests several potential improvements. Some students found the dragging and clicking of electrons repetitive and tiring, especially on non-touchscreen devices, and suggested alternative interaction methods like clicking to grab and click to drop. Others requested features like an "undo" button instead of a full reset, clearer explanations when answers are incorrect or when hints are used, and the inclusion of more graphics or aesthetic enhancements to make the simulation more visually appealing. Some students also expressed interest in seeing applications of these compounds in daily life to increase interest. The designers have noted some of these suggestions, such as increasing the sensitivity of draggable electrons and adding text labels to buttons, and are considering others while also acknowledging potential limitations in implementing certain complex features.
- Beyond methane, does the platform offer resources for understanding covalent bonding in other molecules and ions? Yes, while the "Covalent Bonding 'Dot and Cross' Simulation only methane" focuses specifically on methane, the broader platform "Open Educational Resources / Open Source Physics @ Singapore" provides numerous other resources for learning about chemical bonding using dot and cross diagrams. The linked page showcases simulations and explanations for a variety of molecules including hydrogen (H₂), chlorine (Cl₂), oxygen (O₂), nitrogen (N₂), hydrogen chloride (HCl), water (H₂O), carbon dioxide (CO₂), ammonia (NH₃), hydrogen peroxide (H₂O₂), as well as polyatomic ions like hydroxide (OH⁻), cyanide (CN⁻), nitrite (NO₂⁻), and carbonate (CO₃²⁻). These resources demonstrate covalent bonding in molecules with single, double, and triple bonds, as well as bonding in ions involving coordinate covalent bonds or overall charges.


.png
)