About
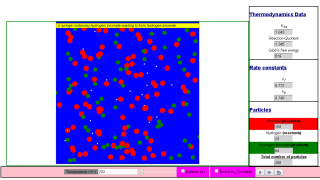
This simulation emulates the gas phase equilibria between hydrogen, bromine and hydrogen bromide molecules.
http://weelookang.blogspot.sg/2014/12/hydrogen-bromine-hydrogen-bromide.html
H2 + Br2 ⇌ 2HBr (∆H = -103 kJ mol-1)
Constants:
kB = R / NA = 1.38060445E23
R = 8.314
NA = 6.022E-23
Parameters:
Hydrogen are white particles. (mass = 2*1.00794 u)
Bromine are red particles. (mass = 2* 79.904 u)
Hydrogen bromide are green particles. (mass = 79.904 + 1.00794 u)
N = 100 molecules
H-H bond: 436 kJ mol-1
Br-Br bond: 193 kJ mol-1
H-Br bond: 366 kJ mol-1
∆H = -103 kJ mol-1
kf = Aexp(-629/8.314/T)
kb = Aexp(-732/8.314/T)
Keq = kf/kb = exp(103/8.314/T)
Fixed relations
v = Math.sqrt(3*8.314/6.022*10E23*T/mass)
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits


![]() Andy Luo Kangshun; Paco; Wolfgang
Andy Luo Kangshun; Paco; Wolfgang
Briefing Doc: ⚛️Hydrogen, Bromine, Hydrogen Bromide Equilibrium Simulation
Source: Excerpts from "Hydrogen, Bromine, Hydrogen Bromide equilibrium JavaScript HTML5 Applet Simulation Model - Open Educational Resources / Open Source Physics @ Singapore | Open Educational Resources / Open Source Physics @ Singapore"
Main Theme: This source describes an interactive simulation designed to help students understand the gas phase equilibrium between hydrogen (H₂), bromine (Br₂), and hydrogen bromide (HBr).
Key Ideas/Facts:
- Equilibrium Reaction: The simulation focuses on the reversible reaction: H₂ + Br₂ ⇌ 2HBr. This reaction is exothermic, meaning it releases heat (∆H = -103 kJ mol⁻¹).
- Visual Representation: The simulation utilizes a visually engaging approach, representing:
- Hydrogen molecules as white particles.
- Bromine molecules as red particles.
- Hydrogen bromide molecules as green particles.
- Particulate Nature of Matter: The simulation emphasizes the particulate nature of matter, showing individual molecules interacting and colliding.
- Factors Affecting Equilibrium: Students can manipulate parameters such as temperature and initial concentrations to observe how these changes shift the equilibrium position.
- Quantitative Aspects: The simulation incorporates calculations of:
- Equilibrium constant (Keq), which is temperature dependent: Keq = exp(103/8.314/T)
- Rate constants for the forward (kf) and reverse (kb) reactions.
- Molecular velocities based on temperature and mass.
- Educational Value: The simulation provides an interactive and visual learning experience, allowing students to explore the dynamic nature of chemical equilibrium and understand how different factors influence the equilibrium position.
Key Quotes:
- "This simulation emulates the gas phase equilibria between hydrogen, bromine and hydrogen bromide molecules."
- "Parameters: Hydrogen are white particles. (mass = 21.00794 u) Bromine are red particles. (mass = 2 79.904 u) Hydrogen bromide are green particles. (mass = 79.904 + 1.00794 u)"
- "Keq = kf/kb = exp(103/8.314/T)"
Overall: The simulation appears to be a valuable educational tool for chemistry students, providing a visually rich and interactive way to learn about the important concept of chemical equilibrium.
Chemistry Equilibrium Study Guide
Quiz
Instructions: Answer the following questions in 2-3 sentences each.
- What is the balanced chemical equation for the reaction simulated in the Hydrogen, Bromine, Hydrogen Bromide equilibrium applet?
- What colors are used to represent hydrogen, bromine, and hydrogen bromide molecules in the simulation?
- What is the enthalpy change (∆H) for the reaction? Is the reaction exothermic or endothermic?
- Define the terms 'kf' and 'kb' in the context of this equilibrium.
- How is the equilibrium constant (Keq) calculated in the simulation?
- What is the relationship between temperature and the equilibrium constant in this reaction?
- Explain how the forward and reverse reaction rates change as the system approaches equilibrium.
- What is the significance of the Boltzmann constant (kB) and Avogadro's number (NA) in the simulation calculations?
- Describe how the simulation visually represents the concept of dynamic equilibrium.
- How does the simulation help in understanding the effect of changing temperature on the equilibrium position?
Answer Key
- The balanced chemical equation is: H2 + Br2 ⇌ 2HBr
- Hydrogen is represented as white, bromine as red, and hydrogen bromide as green.
- ∆H = -103 kJ mol-1. The negative value indicates that the reaction is exothermic.
- kf represents the rate constant for the forward reaction, and kb represents the rate constant for the backward reaction.
- The equilibrium constant (Keq) is calculated as the ratio of the forward rate constant to the backward rate constant: Keq = kf / kb.
- As the temperature increases, the equilibrium constant (Keq) increases in this reaction. This is because the reaction is exothermic.
- Initially, the forward reaction rate is higher. As products form, the reverse reaction rate increases while the forward rate decreases. At equilibrium, the forward and reverse rates become equal.
- The Boltzmann constant (kB) relates the average kinetic energy of particles to the system's temperature. Avogadro's number (NA) is used for converting between molar quantities and the number of particles. These constants are essential for calculating reaction rates and equilibrium constants.
- The simulation shows a continuous interconversion between reactants and products even after equilibrium is reached, demonstrating the dynamic nature of equilibrium.
- The simulation allows users to change the temperature and observe the resulting shift in the equilibrium position. Increasing the temperature favors the endothermic reaction, while decreasing it favors the exothermic reaction.
Essay Questions
- Discuss the concept of chemical equilibrium, including the characteristics of a system at equilibrium.
- Explain Le Chatelier's Principle and how it can be applied to predict the effect of changes in concentration, pressure, and temperature on the equilibrium position of a reaction.
- Analyze the relationship between the equilibrium constant (Keq), the Gibbs Free Energy (ΔG), and the spontaneity of a chemical reaction.
- Describe the factors that affect the rate of a chemical reaction and explain how these factors influence the time it takes for a system to reach equilibrium.
- Evaluate the importance of understanding chemical equilibrium in industrial processes and provide specific examples of how equilibrium principles are applied in the production of important chemicals.
Glossary of Key Terms
TermDefinitionEquilibriumA state in a reversible reaction where the rates of the forward and reverse reactions are equal, resulting in constant concentrations of reactants and products.Equilibrium Constant (Keq)A numerical value that represents the ratio of product concentrations to reactant concentrations at equilibrium.Rate Constant (k)A proportionality constant that relates the rate of a chemical reaction to the concentrations of the reactants.Enthalpy Change (∆H)The heat energy absorbed or released during a chemical reaction at constant pressure. A negative ∆H indicates an exothermic reaction, while a positive ∆H indicates an endothermic reaction.Exothermic ReactionA chemical reaction that releases heat energy into the surroundings.Endothermic ReactionA chemical reaction that absorbs heat energy from the surroundings.Le Chatelier's PrincipleA principle stating that if a change is applied to a system at equilibrium, the system will shift in a direction that counteracts the change.Dynamic EquilibriumA state of equilibrium where the forward and reverse reactions continue to occur at equal rates, even though the net concentrations of reactants and products remain constant.Boltzmann Constant (kB)A physical constant that relates the average kinetic energy of particles in a gas to the temperature of the gas.Avogadro's Number (NA)The number of atoms or molecules in one mole of a substance, equal to 6.022 × 10^23.
Video
https://notebooklm.google.com/notebook/b77e84bc-75bd-4b6c-aee5-6f0ad74d3a42/audio
FAQ: Hydrogen, Bromine, and Hydrogen Bromide Equilibrium Simulation
1. What is the purpose of this simulation?
This simulation visually demonstrates the gas phase equilibrium reaction between hydrogen (H2), bromine (Br2), and hydrogen bromide (HBr) molecules. It allows users to observe how changing parameters like temperature affect the concentrations of each molecule at equilibrium.
2. What is the chemical equation for this equilibrium reaction?
The balanced chemical equation is:
H2 + Br2 ⇌ 2HBr (∆H = -103 kJ mol-1)
This indicates that one molecule of hydrogen reacts with one molecule of bromine to produce two molecules of hydrogen bromide. The reaction is reversible, meaning it can proceed in both the forward and reverse directions. The negative enthalpy change (∆H) indicates the reaction is exothermic, meaning heat is released.
3. How are the different molecules represented in the simulation?
The simulation uses color-coded particles to represent the molecules:
- Hydrogen (H2): White particles
- Bromine (Br2): Red particles
- Hydrogen bromide (HBr): Green particles
4. What parameters can be adjusted in the simulation?
The main parameter that can be adjusted is temperature. By increasing or decreasing the temperature, you can observe how the equilibrium shifts and the relative concentrations of the molecules change.
5. What are the bond energies involved in the reaction?
The simulation provides the following bond energies:
- H-H bond: 436 kJ mol-1
- Br-Br bond: 193 kJ mol-1
- H-Br bond: 366 kJ mol-1
These values represent the energy required to break one mole of each type of bond.
6. What are the equilibrium constants (Keq) and rate constants (kf and kb) used in the simulation?
- The forward rate constant (kf) is calculated using the Arrhenius equation: kf = Aexp(-629/8.314/T), where A is the pre-exponential factor and T is the temperature.
- The backward rate constant (kb) is similarly calculated: kb = Aexp(-732/8.314/T)
- The equilibrium constant (Keq) is the ratio of the forward and backward rate constants: Keq = kf/kb = exp(103/8.314/T)
7. How does the simulation illustrate Le Chatelier's Principle?
Le Chatelier's Principle states that a system at equilibrium will shift to counteract any changes applied to it. This simulation allows you to see this principle in action:
- Increasing temperature: Since the reaction is exothermic, increasing temperature favors the reverse reaction, leading to a decrease in HBr concentration and an increase in H2 and Br2 concentrations.
- Decreasing temperature: Decreasing temperature favors the forward reaction, resulting in a higher HBr concentration and lower H2 and Br2 concentrations.
8. Where can I access and run this simulation?
You can access the simulation by visiting the Open Educational Resources / Open Source Physics @ Singapore website. The simulation runs in a web browser and can be embedded in other webpages.
- Details
- Written by Loo Kang Wee
- Parent Category: 03 Chemistry of Reactions
- Category: 03 Chemical Reactions
- Hits: 7883