v2 20250620 to fix a 2 Cl- ions discharged giving 2 electrons at the anode instead of 4, amount of gas form on both cathode and anode should be the same
"concentrated NaCl": {
negativeIonTypes: [
{
formula: "OH¯",
amount: 2,
},
{
formula: "Cl¯",
amount: 2, // ✅ CHANGE THIS from 6 to 2
},
],
positiveIonTypes: [
{
formula: "H⁺",
amount: 2,
},
{
formula: "Na⁺",
amount: 2, // ✅ CHANGE THIS from 6 to 2
},
],
reactions: [
{
location: "anode", // Where the reaction occurs ("anode" or "cathode")
reactants: ["Cl¯", "Cl¯"], // Array of reactant ion labels
product: "Cl₂", // Product label
electronsTransferred: 2,
},
{
location: "cathode",
reactants: ["H⁺", "H⁺"],
product: "H₂",
electronsTransferred: 2,
},
],
anodeFormula: "2 Cl⁻ (aq) → Cl₂ (g) + 2 e⁻",
cathodeFormula: "2 H⁺ (aq) + 2 e⁻ → H₂ (g)",
//overallFormula: "2 H₂O (l) + 2 Cl⁻ (aq) → H₂ (g) + Cl₂ (g) + 2 OH⁻ (aq) \n "
overallFormula: "2H⁺(aq) + 2Cl⁻(aq) → H₂(g) + Cl₂(g) \n"
}
v2 20250617
Conc NaCl
overallFormula: "2H⁺(aq) + 2Cl⁻(aq) → H₂(g) + Cl₂(g) \n"
old versions was //overallFormula: "2 H₂O (l) + 2 Cl⁻ (aq) → H₂ (g) + Cl₂ (g) + 2 OH⁻ (aq) \n "
v2
Dilute NaCl
1. At the anode, 4 OH- ions attracted at the same time forming only 1 O2 gas (currently every 2 OH- ions attracted form 1 O2 gas)
2. There should be double H2 gas formed instead of O2 gas
3. At the cathode, should be 2 H+ ions form 1 H2 gas instead of 4H+ ions form 1 H2 gas.
Conc NaCl
1. At the anode, should be 2 Cl- ions form 1 Cl2 gas instead of OH- ions form O2 gas.
Other/General settings:
1. Would it be possible to have an additional option where students can remove the showcase of ions that are not involved in the discharge? Which means for eg in dilute NaCl, the Cl- ions will not be shown at the anode.
2. Would it be possible for the ions that was converted to the gas to maybe blink before being converted into the gas?
3. Would it be possible to slow down the movement of the ions or have an option to view the movement in a slow speed and the normal speed?
4. can we have the ions that form the product to have the same colour instead? So for OH- to have the same colour as O2 gas (green), H+ ions to have the same colour as H2 gas(orange), Cl- ions to have the same colour as the Cl2 gas which is red (which means the gas colour for Conc NaCl at the anode need to change)
Credits
['sithu', 'lookang', 'storyboard by sheena']
Credits
['sithu', 'lookang', 'storyboard by sheena']
Sample Learning Goals
Electrolysis Simulation Study Guide
Short Answer Questions (10)
Instructions: Answer each question in 2-3 sentences.
- What are the two types of NaCl solutions used in the Electrolysis Virtual Lab simulation, and how do they differ in terms of ion concentration?
- Describe the visualization of electrolysis reactions in the simulation. What elements are shown, and how are the reactions presented?
- What is the purpose of the dropdown menu for solutions in the simulation? How does it enhance student learning?
- Explain how the simulation accurately represents chemical equations during electrolysis. Give an example of a reaction displayed.
- How do the interactive bubbles in the simulation contribute to understanding gas production during electrolysis?
- Describe the state tracking and scoring system in the simulation. How does it ensure active participation and prevent score manipulation?
- What user interface elements are included in the simulation to enhance usability and clarity for students?
- How does the real-time reaction logic in the simulation ensure an authentic and scientifically accurate experience?
- Describe the ways in which the simulation code can be customized. Provide examples of potential modifications.
- Explain how the simulation bridges the gap between theory and practice in electrolysis.
Answer Key
- The simulation uses dilute NaCl and concentrated NaCl solutions. Dilute NaCl has a lower concentration of Na+ and Cl- ions compared to concentrated NaCl.
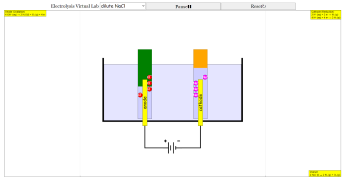
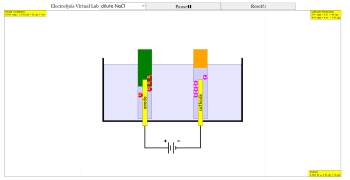
- The simulation visualizes electrolysis by showing the movement of ions (Na+, Cl-, H+, OH-) towards the anode and cathode. Gaseous products (chlorine gas, hydrogen gas) are depicted as bubbles forming and rising.
- The dropdown menu allows users to select different electrolyte solutions, facilitating the comparison of various setups and their respective reactions. It encourages active exploration and reinforces the understanding of electrolyte-specific reactions.
- The simulation dynamically displays oxidation and reduction half-equations at the respective electrodes, along with the overall reaction equation. For example, in concentrated NaCl, the anode reaction is: 2 Cl⁻ (aq) → Cl₂ (g) + 2 e⁻
- The bubbles dynamically rise through the liquid and disappear upon reaching the surface, mirroring real-world observations. This reinforces the concept of gas production as a key outcome of electrolysis.
- The simulation tracks user interactions, awarding points only for correct solution selections ("dilute NaCl" or "concentrated NaCl"). The score limit is capped at 2 per session to prevent repetitive scoring. This encourages thoughtful choices and genuine interaction.
- The simulation features "Play" and "Reset" buttons, a dropdown menu for solution selection, and clear labels for electrodes ("Anode" and "Cathode"). These elements ensure ease of use and enhance the understanding of the simulation setup.
- The backend code constantly checks and processes ion movement, gas production, and electrode-specific reactions based on the chosen solution. This dynamic processing ensures a realistic and scientifically accurate simulation of electrolysis.
- The open-source EJS code allows for customization. Users can add new electrolytes, modify ion concentrations, or adjust the visual elements to tailor the simulation to specific needs.
- The simulation provides a visual and interactive representation of electrolysis, connecting abstract chemical equations with observable phenomena like ion movement and gas formation. This interactive visualization strengthens the understanding of electrolysis principles by directly linking theory to practice.
Essay Questions (5)
- Discuss the advantages and limitations of using virtual lab simulations like the Electrolysis Virtual Lab for teaching chemistry concepts.
- Explain the differences in the electrolysis process and products when using dilute NaCl versus concentrated NaCl solutions. Relate your explanation to the chemical equations involved.
- Analyze the pedagogical effectiveness of the interactive features in the Electrolysis Virtual Lab. How do features like dynamic bubbles and customizable options contribute to student learning and engagement?
- Imagine you are a chemistry teacher planning to incorporate the Electrolysis Virtual Lab into your lesson on electrolysis. Describe a detailed lesson plan outlining the learning objectives, activities, and assessment methods.
- Critically evaluate the potential impact of open-source, customizable simulations like the Electrolysis Virtual Lab on the future of science education. Consider factors like accessibility, adaptability, and community collaboration.
Glossary of Key Terms
Anode: The electrode where oxidation occurs during electrolysis. Cathode: The electrode where reduction occurs during electrolysis. Dilute Solution: A solution containing a relatively low concentration of solute. Concentrated Solution: A solution containing a relatively high concentration of solute. Electrolyte: A substance that conducts electricity when dissolved or molten. Electrolysis: A process that uses an electric current to drive a non-spontaneous chemical reaction. Half-Reaction: One of the two parts of a redox reaction, representing either oxidation or reduction. Ion: An atom or molecule that carries an electric charge. Oxidation: The loss of electrons by a substance in a chemical reaction. Reduction: The gain of electrons by a substance in a chemical reaction. Simulation: A computer model that mimics a real-world process or system. Virtual Lab: A computer-based simulation that allows users to conduct experiments and explore scientific concepts in a safe and interactive environment.
For Teachers
this simulation builder link. This model isn't just a teaching tool—it's a game-changer.In this blog post, we'll explore the simulation's innovative features and how it can transform your chemistry lessons into an unforgettable learning experience. Let's dive in!
Key Features of the Electrolysis Virtual Lab
1. Dynamic Visualization of Electrolysis Reactions
The simulation offers real-time visualization of electrolysis for various solutions, including:
-
Dilute NaCl (Sodium Chloride)
link
https://sg.iwant2study.org/ospsg/index.php/1271 -
Concentrated NaCl
link
https://sg.iwant2study.org/ospsg/index.php/1271
Students can observe how ions move towards the anode and cathode, mimicking real-life laboratory conditions. The gaseous products at each electrode (like chlorine gas and hydrogen gas) are shown bubbling dynamically, enhancing conceptual understanding.
2. Customizable Options for Different Electrolytes
With a simple dropdown menu, users can switch between solutions such as:
 |
| link https://sg.iwant2study.org/ospsg/index.php/1271 |
-
Dilute NaCl
-
Concentrated NaCl
-
Additional electrolytes can be easily added by modifying the source code.
Each option adjusts the reactions displayed and the ionic composition, allowing students to compare and contrast different setups.
3. Accurate Chemical Equations
The simulation dynamically displays the oxidation and reduction half-equations, as well as the overall reaction:
-
Anode Reaction (Oxidation): For example, in concentrated NaCl:
2 Cl⁻ (aq) → Cl₂ (g) + 2 e⁻ -
Cathode Reaction (Reduction): For example,
2 H⁺ (aq) + 2 e⁻ → H₂ (g) -
The overall equation is clearly shown for easy understanding.
This real-time display ensures students can directly link the observed phenomena with the corresponding chemical processes.
4. Interactive Bubbles that React to Liquid Levels
Bubbles representing gas production dynamically rise through the liquid and disappear when they reach the air-liquid boundary. This unique feature mirrors real laboratory observations, reinforcing key concepts about electrolysis and gas production.
 |
| link https://sg.iwant2study.org/ospsg/index.php/1271 |
5. State Tracking and Scoring System
SLS ready for use as Interactive Response HTML5 Item The simulation includes built-in logic to track user interactions, ensuring students:
-
Only score points when correct options are selected (e.g., "dilute NaCl" or "concentrated NaCl").
-
Have a maximum score limit of 2 per session, avoiding repetitive scoring for the same actions.
This feature fosters active participation while preventing exploitation of the scoring system.
6. Seamless User Interface
-
"Play" and "Reset" Buttons: Simple controls allow students to start or restart the simulation anytime.
-
Dropdown Menu for Solutions: Effortless switching between electrolytes.
-
Highlighting Electrodes: Labels such as "Anode" and "Cathode" make it clear which reactions occur where.
7. Real-Time Reaction Logic
The simulation's backend code dynamically checks and processes:
-
The movement of positive and negative ions towards the electrodes.
-
Gas production and its accumulation in test tubes.
-
Reactions based on solution type and electrode polarity.
This ensures an authentic and scientifically accurate simulation.
8. Fully Customizable Code
Built using EJS, the simulation is open-source and highly adaptable. Teachers and developers can:
-
Add new electrolytes.
-
Modify ion concentrations.
-
Adjust the visual appearance to match specific educational needs.
9. Educational Benefits
Bridging Theory and Practice
Traditional textbooks often struggle to connect chemical equations with real-world phenomena. This simulation bridges the gap by visually demonstrating:
-
The movement of ions.
-
Gas formation.
-
Energy flow within the system.
Enhancing Engagement
The interactive nature of the simulation captures students' attention and encourages hands-on learning. Instead of passively reading about electrolysis, they actively explore and manipulate variables.
Promoting Experimentation
Students can "fail safely" in the virtual lab by resetting and trying different electrolytes or observing incorrect setups, fostering a spirit of curiosity and experimentation.
How to Use the Simulation in Your Classroom
-
Demonstrate Electrolysis: Project the simulation during lessons to visually explain electrolysis of dilute and concentrated NaCl.
-
Student Exploration: Assign it as an interactive activity where students predict and observe reactions.
-
Lab Preparation: Use the simulation to prepare students for hands-on experiments, ensuring they understand key concepts beforehand.
Why This Simulation Is a Must-Have for Educators
This EJS-based simulation is not just a tool—it's a transformative learning experience. It makes electrolysis accessible, engaging, and deeply educational. By leveraging the power of interactivity and visualization, educators can inspire a love for chemistry and a deeper understanding of its principles.
Share this simulation with your colleagues, embed it in your teaching, and watch as your students grasp complex concepts with ease.
Get Started Now
Ready to explore? Access the Electrolysis Virtual Lab at iwant2study.org and bring your chemistry lessons to life. Let's make learning viral!
Join the Conversation
Have you used this simulation in your classroom? Share your experiences, tips, or creative ideas in the comments below. Let’s collaborate to make science education unforgettable!
Research
Briefing Doc: Electrolysis Virtual Lab Simulation
Source: Excerpts from "Unlocking Chemistry with EJS: Dive Into the Interactive World , Molecular and Symbolic Representation Electrolysis Simulation by Sheena - Open Educational Resources / Open Source Physics @ Singapore | Open Educational Resources / Open Source Physics @ Singapore"
Main Themes:
- Using interactive simulations to enhance chemistry education, specifically electrolysis.
- The importance of bridging the gap between theoretical concepts and practical visualization.
- Benefits and features of the Electrolysis Virtual Lab simulation built with Easy JavaScript Simulations (EJS).
Most Important Ideas/Facts:
1. Dynamic Visualization:
- The simulation provides real-time visualization of electrolysis reactions for dilute and concentrated NaCl solutions.
- "Students can observe how ions move towards the anode and cathode, mimicking real-life laboratory conditions."
- Dynamically displayed gas production enhances conceptual understanding.
2. Customization:
- Users can switch between different electrolyte solutions using a dropdown menu.
- The source code can be modified to add additional electrolytes.
- This adaptability allows for tailored learning experiences.
3. Accurate Representation:
- The simulation accurately displays chemical equations for oxidation, reduction, and the overall reaction.
- For example, "Anode Reaction (Oxidation): For example, in concentrated NaCl: 2 Cl⁻ (aq) → Cl₂ (g) + 2 e⁻"
- Real-time display links observed phenomena with the corresponding chemical processes.
4. Interactive Features:
- Interactive bubbles react to liquid levels, mirroring real-world observations.
- A state-tracking and scoring system encourages active participation and prevents scoring exploitation.
5. User-Friendly Interface:
- Simple controls ("Play" and "Reset" buttons) and clear labels enhance user experience.
- A dropdown menu facilitates easy switching between electrolytes.
6. Educational Benefits:
- Bridging Theory and Practice: Visual demonstrations help connect chemical equations with real-world phenomena.
- Enhancing Engagement: Interactive nature fosters active learning and exploration.
- Promoting Experimentation: The virtual environment allows for safe experimentation and failure.
7. Open-Source and Adaptable:
- The EJS-based simulation is open-source and customizable.
- Teachers and developers can modify ion concentrations, add electrolytes, and adjust the visual appearance.
Quotes:
- "This simulation bridges the gap by visually demonstrating: The movement of ions. Gas formation. Energy flow within the system."
- "The interactive nature of the simulation captures students' attention and encourages hands-on learning. Instead of passively reading about electrolysis, they actively explore and manipulate variables."
Overall, the Electrolysis Virtual Lab simulation offers a powerful and engaging way to teach and learn about electrolysis. Its interactive features, accurate representation, and customization options make it a valuable resource for chemistry educators.
Electrolysis Virtual Lab FAQ
Video
[text]
https://notebooklm.google.com/notebook/61d1a2e4-d466-45cd-ba42-02a545f56c87/audio
Version:
https://weelookang.blogspot.com/2024/12/unlocking-chemistry-with-ejs-dive-into.html
Other Resources
[text]
end faq
{accordionfaq faqid=accordion4 faqclass="lightnessfaq defaulticon headerbackground headerborder contentbackground contentborder round5"}
- Details
- Written by Loo Kang Wee
- Parent Category: 03 Chemistry of Reactions
- Category: 03 Chemical Reactions
- Hits: 24964