Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits


![]()
 This email address is being protected from spambots. You need JavaScript enabled to view it.; Anne Cox; Wolfgang Christian; Francisco Esquembre
This email address is being protected from spambots. You need JavaScript enabled to view it.; Anne Cox; Wolfgang Christian; Francisco Esquembre
Briefing Document: Rutherford and Thomson Atomic Models
1. Introduction
This document reviews the key themes and concepts presented in the provided source, which focuses on a JavaScript HTML5 applet simulation model designed to illustrate and contrast Rutherford's and Thomson's models of the atom. The document also includes background information on the historical context of these models. The applet is meant to be an interactive educational tool that allows students to visualize and experiment with different atomic models.
2. Key Themes & Concepts
- Historical Context: The document highlights the historical development of atomic theory, focusing on the shift from Thomson's "plum pudding model" to Rutherford's nuclear model.
- Thomson's Plum Pudding Model: J.J. Thomson proposed that the atom was a sphere of positive charge with electrons distributed throughout, "a bit like plums in a Christmas pudding." The model was based on classical (Newtonian) physics and did not account for the existence of protons or neutrons.
- Limitations of Thomson's Model: The model was not universally accepted, and Thomson was never able to develop a stable model. Hantaro Nagaoka rejected it because opposing charges cannot penetrate each other, and proposed a model where electrons orbited positive charge similar to rings around Saturn.
- Rutherford's Gold Foil Experiment: The "Geiger–Marsden experiment(s) (also called the Rutherford gold foil experiment)" was conducted between 1908 and 1913. This experiment, under the direction of Ernest Rutherford, involved firing alpha particles at a thin gold foil. The results of this experiment led to a revolutionary change in our understanding of atomic structure.
- Rutherford's Model: Rutherford concluded that the atom has a small, dense, positively charged nucleus at its center, with a relatively large amount of empty space around it where electrons orbit. This model was supported by the deflection of some of the alpha particles in the experiment.
- Rutherford's rejection of Thomson's model: "Rutherford thus rejected Thomson's model of the atom, and instead proposed a model where the atom consisted of mostly empty space, with all its positive charge concentrated in its center in a very tiny volume, surrounded by a cloud of electrons."
- Rutherford's Interpretation: "In it, the atom is made up of a central charge (this is the modern atomic nucleus, though Rutherford did not use the term 'nucleus' in his paper) surrounded by a cloud of (presumably) orbiting electrons."
- Interactive Simulation: The document describes a JavaScript applet designed to simulate these models, providing students with a hands-on approach to understanding their differences. The simulation features:
- Configurable Parameters: The simulation allows users to adjust parameters like the number and charge distribution of particles, forces and the initial velocity of the alpha particle.
- Visual Representation: It provides a visual representation of the trajectories of alpha particles as they interact with the simulated atoms. This includes the ability to see deflected and un deflected paths.
- Multiple Model Options: The applet includes options to visualize:
- Rutherford's model with a single central charge.
- Rutherford's model with nine tightly packed central charges.
- Thomson's model with nine spread out charges.
- Thomson's model with 79 spread out charges.
- User Control: Users can interact with the simulation by manually changing the initial position and velocity of the alpha particles (He particle) or use the automatic (randomized) setting.
- Mathematical Foundation
- Electrostatic Force: The force between charged particles is calculated using Coulomb's Law: "F = kqQ/r^2" where k is scaled down from the constant used in the nature world for the sake of the computer simulation model.
- Differential Equations: The motion of the particles is governed by ordinary differential equations (ODEs) which determine the particles position over time based on the force applied on it and the particle's mass.
- Mass and Charge Values: The simulation uses defined mass and charge values for helium (alpha) and gold atoms, with "mass of helium atom= 4u" and "charge of helium atom = 2e" and "mass of gold atom = 197u" and "charge of gold atom = 79e"
- Pedagogical Approach: The applet is designed to promote inquiry-based learning. Students can conduct inquiry activities and observe the outcomes, behaving like scientists and "make conclusion based on evidence rather than from reading texts and looking at static pictures."
- Technological Aspects: The simulation is built using EasyJavaScriptSimulation, making it accessible on various devices (Android, iOS, Windows, MacOSX, Linux).
- Other Resources: The document provides links to other related simulations and resources, such as:
- "Understanding Rutherford's gold foil experiment" by Concord Consortium.
- "JJ Thomson wrong model" and "Rutherford experiment result and new model" from kcvs.ca
- The "Rutherford scattering" simulation from PhET.
3. Key Facts & Ideas
- The Geiger-Marsden experiments (gold foil experiment) led to the discovery of the atomic nucleus.
- Rutherford's model of the atom has a small dense nucleus containing most of the mass and all the positive charge, with the electrons orbiting in a large amount of empty space surrounding the nucleus.
- Thomson's plum pudding model was proven to be incorrect by Rutherford's experiments.
- The simulation allows for experimentation with both the Rutherford and Thomson models, including variations to understand the effect of charge distribution.
- The simulation uses computational data and visualizations to aid students in making evidence-based conclusions about atomic structure.
4. Quotes
- "The popular theory of atomic structure at the time of Rutherford's experiment was the 'plum pudding model'."
- "Rutherford thus rejected Thomson's model of the atom, and instead proposed a model where the atom consisted of mostly empty space, with all its positive charge concentrated in its center in a very tiny volume, surrounded by a cloud of electrons."
- "Through conducting inquiry activities on these 4-option computer model, students can obtain computational data (visualise via the histogram or otherwise the He particle trails) and behave like scientists and make conclusion based on evidence rather than from reading texts and looking at static pictures."
5. Conclusion
The provided source details a powerful educational resource for understanding the historical shift in atomic theory from Thomson's plum pudding model to Rutherford's nuclear model. The interactive simulation allows students to explore these models, perform virtual experiments, and develop a deeper understanding of atomic structure and the scientific method. The document includes helpful links to similar models and activities, further reinforcing the concepts covered by the simulation. The applet is designed to be a tool for inquiry-based learning, allowing students to visualize concepts instead of just reading about them.
Rutherford's Atomic Model: A Study Guide
Quiz
Instructions: Answer each question in 2-3 sentences.
- What was the primary goal of the Geiger-Marsden experiments, also known as the Rutherford gold foil experiment?
- Describe the plum pudding model of the atom, and who is credited with its development?
- Why did Hantaro Nagaoka reject the plum pudding model? What was his alternative idea?
- In Rutherford's model, where is the positive charge and most of the mass concentrated within the atom?
- According to the source material, how did Rutherford conduct his experiment to probe the unseen world of the atom?
- What were the main differences between Rutherford's atomic model and the plum pudding model?
- What is the significance of the unified atomic mass number (u) in the computer model’s initial values?
- Explain the force calculation used in the simulation, and describe what the variables represent.
- Briefly explain how the computer model simulates the motion of the charged particles.
- What are the two options for controlling the helium particle in the computer simulation, and how does each one work?
Quiz Answer Key
- The primary goal of the Geiger-Marsden experiments was to investigate the structure of the atom. They sought to determine the distribution of positive charge and mass by observing how alpha particles scattered when directed at a thin metal foil.
- The plum pudding model, developed by Lord Kelvin and J.J. Thomson, proposed that an atom is a sphere of positive charge with electrons embedded throughout, like plums in a pudding. This model assumed the positive charge was distributed uniformly.
- Hantaro Nagaoka rejected the plum pudding model because he believed that opposing charges could not penetrate each other. He proposed instead that electrons orbited a central positive charge, similar to the rings around Saturn.
- In Rutherford's model, the positive charge and most of the mass of the atom are concentrated in a very small, central volume called the nucleus. The rest of the atom is comprised of mostly empty space with electrons orbiting around it.
- Rutherford used alpha particles emitted from a radioactive element as probes. These particles were directed at a thin gold foil, and the scattering pattern of the alpha particles was then observed to determine the structure of the atom.
- Rutherford’s model posited a concentrated positive charge at the center, with most of the atom being empty space, while the plum pudding model proposed a uniformly distributed positive charge throughout a sphere with electrons scattered within. The plum pudding model also lacked a nucleus.
- The unified atomic mass number (u) provides a standard unit for expressing the mass of atoms and subatomic particles. In the simulation, it is used to set the relative masses of the helium and gold atoms, which is important for calculations in the simulation.
- The simulation calculates electrostatic force using F = kqQ/r^2 where ‘k’ is a constant scaled to the simulation, ‘q’ and ‘Q’ are the charges of the two particles, and ‘r’ is the distance between them. This equation determines the strength of attraction or repulsion between charged particles.
- The computer model uses ordinary differential equations to simulate particle motion. These equations calculate changes in position and velocity (dx/dt, dvx/dt, dy/dt, dvy/dt) by determining the net force on a given particle caused by all other charged particles.
- The “auto” option automatically randomizes the y-position of the helium particle each time it is shot toward the gold atom with a constant velocity, while the “manual” option allows the user to drag and position the helium particle and adjust the trajectory for experimentation.
Essay Questions
Instructions: Answer each question using evidence from the provided source. Each essay should contain an introduction, body paragraphs, and conclusion.
- Compare and contrast the plum pudding model and Rutherford's atomic model. How did Rutherford's experiments lead to the rejection of the former and support the latter?
- Discuss the significance of the Geiger-Marsden (Rutherford gold foil) experiments in the context of atomic theory. How did these experiments reshape our understanding of atomic structure?
- Explain the mathematical model used in the simulation. How does this model illustrate the behavior of charged particles within an atom, and what assumptions are made in the simulation?
- Analyze the various interactive models of the Rutherford and Thomson models provided in the source. How do these simulations enhance our understanding of the concepts?
- Rutherford’s model was a significant advancement in atomic theory but was still incomplete. Describe how this model was an improvement on earlier models, and what key limitations were present in this model?
Glossary of Key Terms
- Alpha Particle: A positively charged particle consisting of two protons and two neutrons, emitted from the nucleus of some atoms during radioactive decay.
- Atom: The basic unit of a chemical element, consisting of a central nucleus surrounded by electrons.
- Electrostatic Force: The force of attraction or repulsion between charged objects, described by Coulomb's law (F = kqQ/r^2).
- Geiger-Marsden Experiment: Also known as the Rutherford gold foil experiment, a series of experiments in which alpha particles were fired at a thin gold foil to investigate the structure of the atom.
- Nucleus: The dense, positively charged core of an atom, containing protons and neutrons.
- Ordinary Differential Equations: Equations that involve derivatives of a function, often used to model dynamic systems like particle motion.
- Plum Pudding Model: An early model of the atom proposed by J. J. Thomson, in which electrons are embedded in a sphere of positive charge.
- Rutherford's Atomic Model: A model of the atom proposed by Ernest Rutherford, in which most of the mass and all positive charge are concentrated in a small nucleus, surrounded by mostly empty space with orbiting electrons.
- Unified Atomic Mass Number (u): A standard unit of mass used to express the mass of atoms and subatomic particles, defined as 1/12 the mass of a carbon-12 atom.
Apps

https://play.google.com/store/apps/details?id=com.ionicframework.rutherfordapp857799&hl=en
Introduction
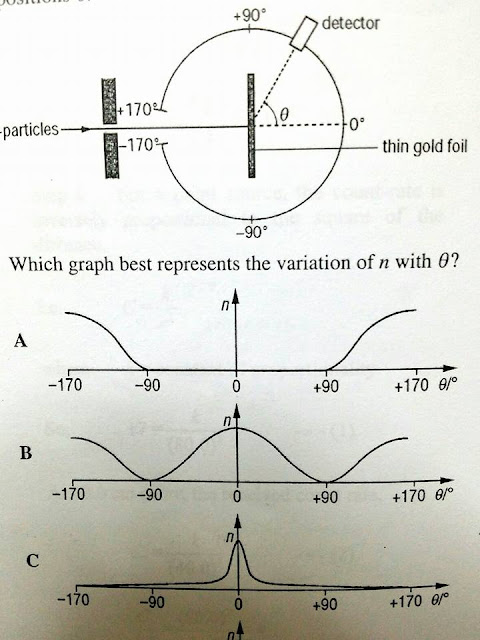
The Geiger–Marsden experiment(s) (also called the Rutherford gold foil experiment) were a landmark series of experiments by which scientists discovered that every atom contains a nucleus where its positive charge and most of its mass are concentrated. They deduced this by measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The experiments were performed between 1908 and 1913 by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherfordat the Physical Laboratories of the University of Manchester.
The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model". This model was devised by Lord Kelvin and further developed by J. J. Thomson. Thomson was the scientist who discovered the electron, and that it was a component of every atom. Thomson believed the atom was a sphere of positive charge throughout which the electrons were distributed, a bit like plums in a Christmas pudding. The existence of protons and neutrons was unknown at this time. They knew atoms were very tiny (Rutherford assumed they were in the order of 10−8 m in radius[1]). This model was based entirely on classical (Newtonian) physics; the current accepted model uses quantum mechanics.
Thomson's model was not universally accepted even before Rutherford's experiments. Thomson himself was never able to develop a complete and stable model of his concept. A Japanese scientist named Hantaro Nagaoka rejected Thomson's model on the grounds that opposing charges cannot penetrate each other.[2] He proposed instead that electrons orbit the positive charge like the rings around Saturn.[3]
Rutherford thus rejected Thomson's model of the atom, and instead proposed a model where the atom consisted of mostly empty space, with all its positive charge concentrated in its center in a very tiny volume, surrounded by a cloud of electrons.
Introduction
The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. Rutherford directed the Geiger–Marsden experiment in 1909, which suggested, upon Rutherford's 1911 analysis, that J. J. Thomson's plum pudding model of the atom was incorrect. Rutherfords new model for the atom, based on the experimental results, contained new features of a relatively high central charge concentrated into a very small volume in comparison to the rest of the atom and with this central volume also containing the bulk of the atomic mass of the atom. This region would be known as the "nucleus" of the atom.
Computer Model Initial Values
mass of helium atom= 4u where u is the unified atomic mass number
mass of gold atom = 197u
charge of helium atom = 2e where e is the electron charge
charge of gold atom = 79e
Forces
Force is calcuated by F = kqQ/r^2 the electrostatic force equation
k = 9.0*0.03 where 0.03 is a factor scaling to scale down/up to fit the x and y dimensions of the computer model, in the nature world k = 9x10^9
q = charge of first particle
Q = charge of second particle
r is the distance that separates the 2 charged particles
Evolution
the ordinary differential equations are
dx[i]/dt = vx[i]
dvx[i]/dt = xForce[i]/m[i]
dy[i]/dt = vy[i]
dvy[i]/dt = yForce[i]/m[i]
where x is the horizontal position of charge particle
i is the array index of all charge particle
t is time
vx is velocity in x direction
xForce is the vector sum of all x-direction forces calculated based on i with all the other [0] to [n] excluding [i] charges particles where n is 80 since it is assume there will be a maximum number of charges of 80.
m is the mass of charged particles
vy is velocity in y direction
yForce is the vector sum of all y-direction forces calculated based on i with all the other [0] to [n] excluding [i] charges particles where n is 80 since it is assume there will be a maximum number of charges of 80.
Auto and Manual Selection of combo-box
auto - automatically randomised the y position of a helium He particle back to the edge of the left screen and shoots in vx as the same initial vx
manual - user can drag on the helium particle to try out their choice of particle y -position and investigate the new trajectory
Rutherford Atom-Model Assumption
The gold atom cannot be moved by fixing the velocity in both x and y idrection as zero all the time.
There is a single particle at the centre of the atom, charge value = 79 u
Rutherford Atom-Model 2 Assumption
The gold atom is made up of 9 individual charge particles that cannot be moved, they are all fixed in their velocities in both x and y idrection to be zero all the time.
There is a group of 9 particles spread out tightly at the centre of the atom, each particle charge is 79u/9. This is a mathematical division as it was too tedious to have 79 particles so to test the model, I just created 9 particles to approximate instead
Thomson Atom-Model configurable Assumption
The gold atom is made up of 9 spread out individual charge particles that can be moved or reposition, they are all fixed in their velocities in both x and y idrection to be zero all the time.
There is a group of 9 particles spread out loosely at the atom, each particle charge is 79u/9. This is a mathematical division as it was too tedious to have 79 particles so to test the model, I just created 9 particles to approximate instead
Thomson Atom-Model configurable each atom Assumption
The gold atom is made up of 79 spread out individual charge particles that can be moved or reposition for exploration and learning, they are all fixed in their velocities in both x and y idrection to be zero all the time.
There is a group of 79 particles spread out loosely at the atom, each particle charge is 79u/79 = 1u. This is the best computer model for Thomsons model of the atom
Experimental basis for Rutherford model
Through conducting inquiry activities on these 4-option computer model, students can obtain computational data (visualise via the histogram or otherwise the He particle trails) and behave like scientists and make conclusion based on evidence rather than from reading texts and looking at static pictures.
Rutherford overturned Thomsons model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny and heavy nucleus. Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. If Thomson was correct, the beam would go straight through the gold foil. Most of the beams went through the foil, but a few were deflected.
Rutherford presented his own physical model for subatomic structure, as an interpretation for the unexpected experimental results. In it, the atom is made up of a central charge (this is the modern atomic nucleus, though Rutherford did not use the term "nucleus" in his paper) surrounded by a cloud of (presumably) orbiting electrons. In this May 1911 paper, Rutherford only committed himself to a small central region of very high positive or negative charge in the atom.
Video
Rutherford's Experiment: Nuclear Atom by uploaded by HerrPingui
Rutherford experiment animation by owigger
Rutherford Gold Foil Experiment - Backstage Science
Rutherford Scattering by xmphysics
Version
Other Resources
- Understanding Rutherford's gold foil experiment by Concord Consortium. Rutherford shot alpha particles at atoms to see if the positive part of atoms would interact strongly with the positve alpha particles. Explore how concentrating positive charge affects the electirc field generated by that charge and how that field affects the path of the positive alpha particles.
- JJ Thomson wrong model http://www.kcvs.ca/site/
projects/physics_files/ rutherford/thomson_model.swf - Rutherford experiment result and new model http://www.kcvs.ca/site/
projects/physics_files/ rutherford/nuclear_atom.swf - https://phet.colorado.edu/en/simulation/rutherford-scattering by PhET
Assessment
 |
| assessment question |
Frequently Asked Questions: Atomic Models and Rutherford's Experiment
- What was the prevailing model of the atom before Rutherford's experiments, and who developed it? The prevailing model was the "plum pudding model," developed by Lord Kelvin and further refined by J.J. Thomson. Thomson, who discovered the electron, proposed that the atom was a sphere of positive charge with negatively charged electrons embedded within it, like plums in a pudding. The model was based on classical physics and did not account for protons or neutrons, as they were unknown at the time.
- What was the key experiment conducted by Geiger and Marsden under Rutherford's direction, and what was its purpose? The Geiger-Marsden experiment, also known as the Rutherford gold foil experiment, involved firing alpha particles at a thin gold foil. The purpose of the experiment was to test the validity of the plum pudding model of the atom. If Thomson's model were correct, the alpha particles were expected to pass through the gold foil with minimal deflection.
- What were the unexpected results of Rutherford's gold foil experiment? While most alpha particles passed through the gold foil with minimal or no deflection, a small fraction of the particles were deflected at very large angles, some even bouncing back. This was inconsistent with the plum pudding model, where the positive charge was considered to be spread out and thus would not cause significant deflection of the alpha particles.
- How did Rutherford interpret the results of the gold foil experiment, and what model did he propose? Rutherford interpreted the scattering results to mean that the positive charge and most of the mass of an atom were concentrated in a very small central region, which he called the nucleus. He proposed that the atom was mostly empty space, with electrons orbiting the nucleus. This model contrasted sharply with the plum pudding model by centralizing the atom's positive charge in a tiny volume and introducing the idea of mostly empty space within the atom.
- How does the provided simulation model represent the Rutherford and Thomson models? The provided simulation model allows for a comparison between the Rutherford and Thomson models. In the Rutherford model simulation, there is a concentrated positive charge at the center of the atom. This representation highlights a central nucleus with a positive charge and the bulk of the mass concentrated in a tiny volume. In the Thomson configurable model, the positive charge is represented by many particles spread more loosely within the atom, with electrons included. The simulation allows for varying the charge distribution to approximate the Thomson model to see the experimental results from a new perspective.
- How does the simulation allow users to explore the scattering of alpha particles? The simulation allows users to observe the trajectory of alpha particles as they interact with different atomic models. Users can control the initial position and velocity of the alpha particles, and they can either randomize the alpha particle launching with the auto option or manually drag the alpha particle to investigate different starting points. By visualizing the scattering patterns, they can gain a better understanding of the forces acting on the alpha particles, the impact of charge distribution, and understand how Rutherford came to his model. Histograms track the paths taken.
- What mathematical equations govern the forces and particle motion in the simulation? The simulation calculates the electrostatic force between particles using Coulomb's Law, given by F = kqQ/r^2, where k is a scaling factor of the electrostatic constant, q and Q are the charges of the interacting particles, and r is the distance between them. The movement of the particles is simulated using ordinary differential equations (ODEs) based on Newtonian mechanics. The ODE's model the relationship between position, velocity, acceleration, mass, and the forces being acting on each particle.
- What is the key takeaway from comparing the simulations of the Rutherford and Thomson models? By comparing the two models, users can see how Rutherford's nuclear model is more consistent with the experimental data from the gold foil experiment than the plum pudding model. Specifically, when the central charge is concentrated, there is a higher probability for greater deflections compared to when the central charge is spread out. This visualization emphasizes the importance of a concentrated positive charge, the nucleus, in order to produce the large deflections that were observed in the experiment. Through experimentation of the model and looking at the results of the trajectory paths of the alpha particles, learners can build and make their own scientific conclusion on what atomic model was likely correct.
- Details
- Written by Loo Kang Wee
- Parent Category: 06 Modern Physics
- Category: 02 Nuclear
- Hits: 22225


.png
)





