About
Brownian motion
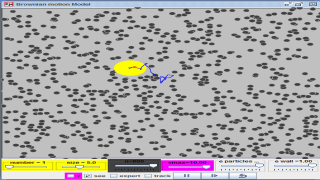
This is a simulation of brownian motion of a particle that collides with a large set of smaller particles which move with uniform motion in different random directions.
For more info:
http://weelookang.blogspot.sg/2010/06/ejs-open-source-brownian-motion-gas.html
Original Author:
Simulación preparada por Francisco Esquembre para el libro
Creación de Simulaciones Interactivas en Java.
Aplicación
a la Enseñanza de la Física
(C) Pearson Educación 2004.
Modified by Fu-Kwun Hwang
http://www.phy.ntnu.edu.tw/ntnujava/
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits


 Francisco Esquembre; Fu-Kwun Hwang; lookang
Francisco Esquembre; Fu-Kwun Hwang; lookang
Briefing Document: Open Educational Resources / Open Source Physics @ Singapore
1. Overview:
This document is a compilation of interactive physics simulations and resources hosted by Open Educational Resources / Open Source Physics @ Singapore. It focuses heavily on simulations created using Easy JavaScript Simulation (EJS) and designed to be used across a range of educational levels, from primary school to junior college. The core themes revolve around using interactive models to visualize and understand complex physics concepts, with an emphasis on open-source accessibility.
2. Main Themes:
- Interactive Simulations for Learning: The website's primary focus is the use of interactive simulations as a tool for learning physics and related concepts. The vast majority of the listed items are simulations covering a broad spectrum of physics topics.
- Brownian Motion and Diffusion: A significant theme, highlighted by the title "Brownian Motion Gas Model", is the exploration of Brownian motion and diffusion, using simulations to show how molecules move randomly and how factors like temperature and mass influence their movement. The text states: "Brownian motion provides evidence of the movement of molecules. Pollen grains in water and smoke particles in air are observed to be in constant random motion due to collisions by the unseen fastmoving molecules of water and air respectively".
- Open Source and Accessibility: The materials heavily promote the use of open-source tools and resources, especially Easy JavaScript Simulation (EJS), making these educational tools accessible for various platforms like Windows, MacOSX, Linux, Chromebooks, and even mobile devices. The license is Creative Commons Attribution-Share Alike 4.0 Singapore License indicating the availability for free use.
- Broad Range of Topics: The resources cover an extensive range of physics concepts including:
- Kinematics (motion, projectile motion, velocity, acceleration)
- Newtonian Mechanics (forces, energy, momentum, collisions)
- Oscillations and Simple Harmonic Motion (springs, pendulums)
- Gravitation (fields, escape velocity, satellites)
- Waves (superposition, diffraction, single slit, multiple slit)
- Thermodynamics (Brownian Motion, Diffusion)
- Electromagnetism (electric fields, magnetic fields)
- Optics (light and shadow, ray tracing, reflection, resolution)
- Atomic Physics (Hydrogen spectrum, X-ray crystallography)
- Modern physics (relativity, wave-particle duality)
- Cross-Curricular Applications: While primarily focused on physics, the site includes resources for other subjects such as Mathematics (geometry, trigonometry, calculus, estimation), Chemistry (electrolysis, chemical bonding), Geography (urbanization, transit planner) and even language arts (word blending games, interactive story templates). This indicates a holistic approach to using interactive simulations in education.
- Inquiry-Based Learning: Many simulations encourage students to explore concepts by interacting directly with the model and observing the results; for example the site includes simulations where users can 'add a drop of dye anywhere in the container, and watch it diffuse through the water'.
3. Important Ideas and Facts:
- Brownian Motion as Evidence of Molecular Movement: The resource emphasizes that Brownian motion serves as direct evidence of the random movement of molecules, even those too small to see. This motion is caused by collisions with the surrounding molecules.
- Diffusion and Factors Affecting It: The simulations on diffusion highlight the impact of temperature, molecular mass, and pore size on the rate of diffusion. This aligns with our understanding of kinetic theory.
- EJS (Easy JavaScript Simulation) as a Key Tool: EJS is not just mentioned; it’s pervasive. The site relies heavily on simulations created with EJS, suggesting it's a crucial tool for creating these interactive resources.
- Use of Simulations as a Pedagogical Tool: The site and its accompanying content indicates a move towards the use of simulations as a central tool in educational environments.
- Variety of Educational Levels Targeted: The range of simulations means they can be utilized in diverse educational settings, starting from primary school right up to Junior College.
- Adaptable Simulations: The resources are offered across multiple platforms. The emphasis on being able to run these interactive programs on both computers and phones means that they can be deployed in a variety of different educational environments.
4. Quotes from the Source:
- "This is a simulation of brownian motion of a particle that collides with a large set of smaller particles which move with uniform motion in different random directions." - This describes the core simulation model.
- "Brownian motion provides evidence of the movement of molecules. Pollen grains in water and smoke particles in air are observed to be in constant random motion due to collisions by the unseen fastmoving molecules of water and air respectively" - Clarifies the observational significance of Brownian motion
- "Add a drop of dye anywhere in the container, and watch it diffuse through the water." - Example of an interactive student exercise
- "Surprisingly similar random movement between Brownian and diffusion." - Highlights the inherent relationship between the two.
- "Explore the role of temperature on the rate of diffusion....Compare the diffusion rates at low, medium and high temperatures." - Examples of learning tasks based on the diffusion interactive.
5. Additional Observations:
- Extensive List: The sheer volume of resources is notable, covering 1169 articles, reflecting the dedication of the creators and users of the platform.
- Community Driven: The blog posts and conference papers cited suggest a community of educators and developers actively using and improving the simulations and resources
- Focus on Interactive Learning: The emphasis on interactive models aligns with constructivist learning principles, where students learn by doing and exploring.
- Multilingual Access: The presence of translations suggests an attempt to make the resources more accessible to different user groups
6. Conclusion:
This collection of resources represents a significant contribution to open educational resources, especially in the field of physics education. The focus on interactive simulations, built using open-source tools like EJS, provides a valuable platform for teachers and students to explore complex scientific concepts. The breadth of topics covered, from fundamental mechanics to modern physics, and even topics in chemistry and mathematics make this a rich resource for anyone interested in physics education. The prominence of Brownian motion and diffusion simulations underscores its importance as a fundamental concept for visualizing the movement of molecules.
Brownian Motion & Diffusion Study Guide
Quiz
- What is Brownian motion and what causes it?
- How does temperature affect the rate of diffusion?
- How does molecular mass affect the rate of diffusion?
- What does it mean for a membrane to be selectively permeable?
- Give an example of a molecule that can easily cross a cell membrane and in which direction it moves with respect to a cell that is in an oxygen-rich environment.
- What role do collisions play in Brownian motion?
- What is the relationship between Brownian motion and diffusion?
- How is the simulation of Brownian motion in the provided source described?
- Describe how to use the simulation model to observe the movement of a dye in water.
- What are some of the other interactive simulation models made available on this site?
Quiz Answer Key
- Brownian motion is the random movement of particles, such as pollen in water or smoke in air. It is caused by the collisions of these particles with the smaller, fast-moving molecules of the surrounding medium (like water or air).
- Higher temperatures increase the rate of diffusion because molecules move faster and have more kinetic energy. This allows them to spread out more quickly.
- Lighter molecules diffuse more rapidly than heavier molecules, as their lower mass means they will move faster when imparted with the same amount of kinetic energy.
- A selectively permeable membrane allows some molecules to pass through while blocking others, often based on size, charge, or other properties.
- Oxygen can freely cross a cell membrane. In an oxygen-rich environment outside the cell and an oxygen-poor environment inside the cell, oxygen molecules will move into the cell, along their concentration gradient.
- Collisions between the larger visible particles and the smaller, invisible molecules are the driving force behind Brownian motion. These collisions impart momentum and result in the random movement of the larger particles.
- Brownian motion provides evidence of the movement of molecules. In this way it explains how diffusion happens, as it is the continuous bombardment of molecules that causes them to move and disperse.
- The simulation models Brownian motion as the movement of a single, larger particle colliding with numerous smaller particles that move with uniform motion in random directions.
- In the simulation, a drop of dye can be added to the water. By clicking in the model, the user can observe the dye molecules diffusing through the water and can trace an individual molecule to see its random path.
- Other simulation models on the site include those related to springs, pendulums, gravity, collisions, electromagnetism, optics, and various mathematical concepts. There are also interactive simulations and games for diverse topics, some in primary science.
Essay Questions
- Discuss how the simulation of Brownian motion in the source provides a model for understanding the kinetic theory of matter. Consider how different parameters of the simulation could be changed to model different physical situations.
- Explain the relationship between Brownian motion and diffusion, and discuss how the provided interactive simulations help illustrate these concepts. Provide specific examples of what can be learned by experimenting with each of the simulations.
- Describe how a cell membrane's selective permeability affects the diffusion of different molecules, and why this is important for cell function. Relate this to the interactive membrane model provided.
- Using examples from the provided source and the real world, analyze the significance of Brownian motion as evidence for the existence of molecules and their constant movement.
- Explore the ways that the interactive simulations and other resources on this website could enhance a high school student's understanding of physics and mathematics, as well as other academic subjects.
Glossary of Key Terms
- Brownian motion: The random movement of particles suspended in a fluid (a liquid or a gas) resulting from their collision with the fast-moving molecules of the fluid.
- Kinetic Theory of Matter: A theory that explains the macroscopic properties of matter in terms of the microscopic motion of its constituent particles. It states that all matter is composed of particles in constant motion.
- Diffusion: The net movement of particles from a region of higher concentration to a region of lower concentration, driven by the random motion of molecules.
- Selectively permeable membrane: A biological membrane that allows certain molecules or ions to pass through it by means of active or passive transport.
- Molecular mass: The mass of a molecule, typically measured in atomic mass units (amu) or Daltons (Da).
- Concentration Gradient: The gradual change in the concentration of a solute in a solution across a distance. Molecules tend to move from areas of high concentration to areas of low concentration until equilibrium is reached.
- Pore Size: The size of openings or channels in a membrane, which affects which molecules can pass through.
- Kinetic Energy: The energy possessed by an object due to its motion.
surprisingly similiar random movement between brownian and diffusion.
Key inquiry question: How can we explain the effects of heat gain or heat loss on matter?
2. Brownian motion
• Brownian motion provides evidence of the movement of molecules. Pollen grains in water and smoke particles in air are observed to be in constant random motion due to collisions by the unseen fastmoving molecules of water and air respectively
Other Resources
http://mw.concord.org/nextgen/interactives/
Add a drop of dye anywhere in the container, and watch it diffuse through the water.
Click in the model to add a drop of dye. Watch how the molecules move through the water. Trace an individual molecule to see how it moves through the liquid.
http://lab.concord.org/embeddable.html#interactives/sam/diffusion/1-dropping-dye-on-click.json
How does temperature affect the rate of diffusion?
Explore the role of temperature on the rate of diffusion. Set the temperature, then remove the barrier, and measure the amount of time it takes the blue molecules to reach the gas sensor. When the gas sensor has detected three blue molecules, it will stop the experiment. Compare the diffusion rates at low, medium and high temperatures. Trace an individual molecule to see the path it takes.
http://lab.concord.org/embeddable.html#interactives/sam/diffusion/2-temperature.json
How does molecular mass affect the rate of diffusion?
Explore the role of molecular mass on the rate of diffusion. Select the mass of the molecules behind the barrier. Remove the barrier, and measure the amount of time it takes the molecules to reach the gas sensor. When the gas sensor has detected three molecules, it will stop the experiment. Compare the diffusion rates of the lightest, heavier and heaviest molecules. Trace an individual molecule to see the path it takes.
http://lab.concord.org/embeddable.html#interactives/sam/diffusion/3-mass.json
How does pore size affect the diffusion of different molecules?
Biological membranes are selectively permeable; some molecules can cross while others cannot. One way to affect this is through pore size. Change the pore size with the slider to change the permeability of the membrane to the different types of molecules. Trace an individual molecule to see the path it takes.
Cell membranes are composed of two layers of phospholipids (a phospholipid bilayer). Some molecules are capable of crossing this membrane directly, without use of specific membrane channels.
Oxygen and carbon dioxide are two molecules that can freely cross the cell membrane. In aerobic cells, oxygen is necessary for cell functioning and carbon dioxide is produced as a waste molecule. Hence, the cell “wants” oxygen to enter and carbon dioxide to leave. But molecules don’t move only in one direction–they diffuse randomly across the membrane.
Set up the model with high oxygen and low carbon dioxide outside the cell and low oxygen and high carbon dioxide inside the cell. In which direction do the oxygen and carbon dioxide molecules move?
http://lab.concord.org/embeddable.html#interactives/sam/diffusion/5-permeable-membrane.json
How does pore size affect the diffusion of different molecules?
Biological membranes are selectively permeable; some molecules can cross while others cannot. One way to affect this is through pore size. Change the pore size with the slider to change the permeability of the membranes to the different types of molecules. Trace an individual molecule to see the path it takes.
Video
https://www.youtube.com/watch?v=gPMVaAnij88 by STEM Learning
Versions:
Other resources
http://www.phy.ntnu.edu.tw/ntnujava/index.php?topic=1121.0 simplied flu spreading model by Fu-Kwun Hwang
Frequently Asked Questions About Brownian Motion, Diffusion, and Related Concepts
- What is Brownian motion and how does it provide evidence for the movement of molecules? Brownian motion is the random movement of larger particles (like pollen grains in water or smoke particles in air) that are bombarded by smaller, unseen molecules. The erratic motion of these larger particles provides direct visual evidence that molecules are constantly moving and colliding with each other, even if the molecules themselves are too small to see.
- How does temperature affect the rate of diffusion? Temperature plays a significant role in the rate of diffusion. As the temperature of a substance increases, the kinetic energy of its molecules also increases. This higher energy causes molecules to move faster and collide more frequently, resulting in a faster rate of diffusion. Conversely, lower temperatures slow down molecular movement and diffusion.
- How does molecular mass affect the rate of diffusion? Molecular mass has an inverse relationship with diffusion rate. Lighter molecules move faster at the same temperature than heavier molecules. Therefore, lighter molecules will diffuse more quickly, while heavier molecules will diffuse more slowly due to their lower kinetic energy at the same temperature.
- How does pore size affect diffusion across a membrane? The size of pores in a membrane directly impacts which molecules can pass through. Larger pores allow a wider range of molecules to diffuse through, while smaller pores restrict passage to smaller molecules. This property of selective permeability is critical in biological systems for regulating the movement of substances in and out of cells.
- How do oxygen and carbon dioxide move across cell membranes? Oxygen and carbon dioxide are small, nonpolar molecules that can freely diffuse across the cell membrane, which is a phospholipid bilayer. In the presence of concentration gradients, oxygen tends to diffuse from areas of high concentration (outside the cell in aerobic cells) to areas of low concentration (inside the cell), while carbon dioxide diffuses from high concentrations inside the cell to lower concentrations outside. This movement is driven by the concentration gradient and the random movement of molecules.
- What are some examples of interactive simulations available for studying these concepts? There are numerous interactive simulations available online that illustrate Brownian motion, diffusion, and related topics. For example, there are simulations that show the random motion of particles in Brownian motion, simulations that allow you to visualize the diffusion of a dye in water, and simulations that demonstrate the effects of temperature, mass, and pore size on diffusion rates. These simulations often allow users to trace the paths of individual molecules. Additionally, there are various other simulations available dealing with topics like mechanics (e.g., spring-mass systems, projectile motion), waves, electromagnetism, and even simple games and quizzes.
- What is the significance of the Easy JavaScript Simulation (EJS) environment mentioned in the sources? The Easy JavaScript Simulation (EJS) environment is a tool that allows educators and researchers to create and share interactive simulations of various scientific and mathematical concepts. These simulations can be embedded in web pages or other educational platforms, making it easy to access and use them for learning purposes. The EJS framework is also open-source, which allows for community collaboration and improvement of these resources.
- How do these simulations enhance learning about complex scientific concepts? Interactive simulations enhance learning by allowing students to visualize abstract concepts, manipulate variables, and observe the direct consequences of their actions. These visual tools are more engaging and enable learners to explore and grasp complicated scientific ideas like diffusion and molecular motion in a more intuitive way that is not possible with traditional teaching methods. Furthermore, the ability to customize simulations and integrate them with other educational resources promotes a more personalized and effective learning experience.
- Details
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 13255