About

Molecular Dynamics JS Performance Model
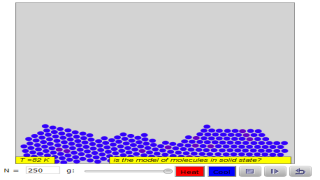
The Molecular Dynamics JavaScript Performance Model computes the trajectory of particles acted on by a Lennard-Jones force. This simulation is designed to test the speed of JavaScript for a computationally intensive model. The user can vary the number of particles, the number of frames per second, and the number of Verlet steps between frames. The actual number of Verlet steps per frame is shown.
Note: If the model becomes unstable, reset the model and reduce the computational timestep dt.
Credits:
The Molecular Dynamics JavaScript Performance Model was developed by Wolfgang Christian and Francisco Esquembre using version 5 of the Easy Java Simulations (EJS 5) modeling tool. Although EJS is a Java program, EJS 5 creates stand alone JavaScript programs that run in almost any PC or tablet browser. Information about EJS is available at: <http://www.um.es/fem/Ejs/> and in the OSP ComPADRE collection <http://www.compadre.org/OSP/>.
http://weelookang.blogspot.sg/2014/10/ejss-three-state-of-matter-model.html
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits


 Wolfgang Christian; Francisco Esquembre; lookang
Wolfgang Christian; Francisco Esquembre; lookang
Briefing Document: Molecular Dynamics Simulation and the Kinetic Model of Matter
1. Overview
This document reviews a collection of resources centered around a JavaScript HTML5 applet simulation model, specifically the "Molecular Dynamics JavaScript Performance Model." The primary focus is on visualizing and understanding the Kinetic Model of Matter, a fundamental concept in physics and chemistry. The model is part of a larger suite of interactive resources available through the Open Educational Resources / Open Source Physics @ Singapore initiative. This initiative seeks to provide engaging, accessible, and computationally intensive simulations for educational purposes.
2. Main Themes and Concepts
- Kinetic Model of Matter: The core theme is the explanation of macroscopic properties of matter (like temperature, state, and volume) through the behavior of microscopic particles (atoms/molecules). This includes:
- States of Matter: The simulation aims to illustrate the differences between solids, liquids, and gases at the molecular level, relating these states to the movement and arrangement of particles and the forces between them.
- Particle Motion: The simulation and accompanying resources emphasize that molecules are in constant, random motion, and that this motion is directly related to temperature.
- Energy Transfer: The document addresses how heat is transferred as kinetic energy, and not as a "substance." It highlights that heat transfer causes changes in temperature, state, volume, or even the substance itself.
- Internal Energy: The distinction between internal energy (the total kinetic and potential energy of the particles) and heat transfer is emphasized, addressing a common student misconception.
- Expansion and Contraction: The model illustrates how heating or cooling matter increases or decreases molecular motion, causing expansion or contraction respectively.
- Pressure: The relationship between gas pressure and the collisions of gas molecules with the container walls is explained.
- Molecular Dynamics: The simulation uses a Lennard-Jones potential to model the interactions between particles, an approximation of interatomic forces. It employs the Verlet algorithm to calculate particle trajectories based on Newton's laws of motion.
- Computational Performance: A key design aspect of the "Molecular Dynamics JavaScript Performance Model" is its use as a tool to test the performance of JavaScript in handling computationally intensive models. The model is designed to be scalable, allowing users to adjust parameters such as the number of particles, frames per second, and Verlet steps per frame.
- Open Educational Resources: This project is part of a larger initiative to make educational resources open, free and accessible.
3. Key Ideas and Facts
- Simulation Details:
- The model computes particle trajectories based on Lennard-Jones forces, truncated at 3 molecular diameters.
- The Verlet algorithm approximates motion based on Newton's laws.
- The user can modify parameters such as: the number of particles, frames per second, and number of Verlet steps per frame.
- The simulation runs on a wide variety of platforms including mobile phones and laptops.
- The time step (dt) is crucial; if too large, it can cause instability.
- Misconceptions Addressed: The document explicitly outlines common student misconceptions regarding heat, particle motion, and diffusion, including:
- The incorrect notion that "heat" and "cold" are substances that flow in and out of materials.
- The misunderstanding that objects "contain" heat, rather than internal energy.
- The idea that individual particles move randomly, instead of constant motion broken by collisions.
- The misunderstanding of diffusion as particles "seeking" lower concentration gradients.
- The false assumption that particulate motion stops when uniform distribution is achieved.
- Learning Objectives: The resources target students from primary to junior college levels, aiming to enhance their understanding of:
- The three states of matter and their properties.
- The concept of matter as made of particles in constant motion.
- The effects of heat gain/loss on matter, including changes in temperature, state, and volume.
- The connection between molecular behavior and macroscopic phenomena.
- The application of these concepts in real-world scenarios.
- Thermodynamics and the law of conservation of energy.
- Supporting Materials: In addition to the primary simulation, there are other embedded applets and links to further explore specific areas:
- Links to simulations focusing specifically on how molecules move in each state of matter (solid, liquid and gas).
- A model showing how the strength of intermolecular attractions affects the state of matter.
- A model demonstrating how energy input causes changes of state.
- Links to other types of simulations including those from PhET and NetLogo.
- Wordwall games for reinforcement of concepts.
4. Key Quotes from Source:
- "The Molecular Dynamics JavaScript Performance Model computes the trajectory of particles acted on by a Lennard-Jones force. This simulation is designed to test the speed of JavaScript for a computationally intensive model."
- "When a substance gains or loses heat, the substance may change its temperature, change its state, change its volume or change to a different substance."
- "Internal energy of a body consists of the total kinetic energy and potential energy of the particles in the body. Changes in a body or a substance due to heat gain or heat loss may be explained by the change in its internal energy."
- "Students have difficulty understanding that heat is energy in transit."
- "Molecules are in constant motion."
5. Educational Implications
The resources provided demonstrate a commitment to interactive, simulation-based education. By visualizing abstract concepts like molecular motion and energy transfer, these models can help students develop a more intuitive and accurate understanding of the Kinetic Model of Matter. The focus on addressing common misconceptions highlights the importance of targeted instruction. The wide range of accompanying resources provides multiple approaches for different learners.
6. Conclusion
The "Molecular Dynamics JavaScript Performance Model" and associated materials offer a valuable tool for teaching and learning about the Kinetic Model of Matter. The simulation itself allows for a deep dive into the microscopic world, while the supporting information provides the necessary framework and addresses common difficulties with the content. The initiative highlights the benefits of open-source educational resources for fostering engaging and effective learning experiences.
Kinetic Model of Matter Study Guide
Quiz
Answer each question in 2-3 sentences.
- What is the Lennard-Jones force and how is it used in the Molecular Dynamics JavaScript Performance Model?
- Explain the relationship between heat transfer and a substance's change in temperature, state, or volume, as described by the kinetic model.
- According to the kinetic model, how does the internal energy of a body change?
- Describe how the kinetic model explains the pressure exerted by a gas.
- What is the main difference between the movements of particles in solids, liquids and gases?
- How does the provided text address the common misconception that objects contain a quantity of heat?
- What is the difference between the heat transfer (Q) and internal energy (U) of an object?
- According to the text, how is diffusion related to the constant motion of particles?
- How does the kinetic model explain the expansion and contraction of matter (with the exception of water)?
- What are some of the practical applications of understanding the kinetic model of matter?
Quiz Answer Key
- The Lennard-Jones force is an interatomic potential used to calculate the forces between particles. In the model, it dictates how the particles interact and move, allowing for a simulation of molecular dynamics based on these forces.
- Heat transfer can cause a substance to change its temperature, state (solid, liquid, or gas), or volume. This is because the kinetic model explains that adding heat increases kinetic energy of molecules, leading to these changes.
- The internal energy of a body changes with alterations in the total kinetic energy and potential energy of its constituent particles. When heat is added, both kinetic and potential energy can increase, leading to an increase in the body's internal energy.
- The kinetic model explains that gas pressure results from the numerous collisions of gas molecules with the walls of their container. These collisions exert a force per unit area, which we perceive as pressure.
- In solids, particles are closely packed and vibrate in fixed positions. In liquids, particles have more freedom to move and slide past each other. In gases, particles are very far apart, move randomly at high speeds, and are unconstrained by positions or interparticle forces.
- The text clarifies that heat is not a substance stored within objects, but is the transfer of energy due to collisions of particles. The concept of internal energy (U) represents stored potential energy in the object, which is different from heat (Q).
- Heat transfer (Q) refers to the energy exchanged between objects due to temperature differences, while internal energy (U) represents the total energy stored within an object's particles (kinetic and potential). They are distinct concepts; one relates to energy flow, the other to energy stored.
- Diffusion is caused by the system of particle collisions that spread particles from high-concentration areas to low-concentration areas. While individual particles move in a straight path at a constant speed until they collide with something, the collection of those collisions drives diffusion.
- The kinetic model explains that as the temperature of matter increases, thermal energy is transferred to the molecules, increasing their kinetic energy, and causing them to move more and therefore expand. The opposite occurs when matter is cooled and molecules lose kinetic energy, causing matter to contract.
- Practical applications of the kinetic model of matter include controlling and using heat transfer in appliances and machines, understanding the behavior of gases, and designing materials with specific thermal properties. It also helps to understand changes in state in everyday life.
Essay Questions
- Discuss how the kinetic model of matter can be used to explain the differences in the macroscopic properties of solids, liquids, and gases. Include discussion of particle movement, spacing, and inter-particle forces.
- Analyze the ways in which the Molecular Dynamics JavaScript Performance Model simulates the behavior of particles using the Lennard-Jones force and Verlet algorithm. How does this simulation enhance the understanding of the kinetic model?
- Evaluate common misconceptions about heat and internal energy, and explain how the kinetic model addresses these misconceptions with a focus on transfer and energy states.
- Explain how the kinetic model relates temperature, pressure, volume, and energy transfer.
- Describe how the knowledge of the kinetic model and the behavior of particles informs our use of energy to affect changes of state, expansion, and contraction and how it might be used to address common issues and challenges in engineering and technology.
Glossary of Key Terms
Kinetic Model of Matter: A model that explains the macroscopic properties of matter and changes in its state in terms of its microscopic molecular structure and behavior.
Lennard-Jones Force: An interatomic potential that describes the interaction between two non-bonding atoms or molecules and used to calculate the forces between particles in the Molecular Dynamics model.
Verlet Algorithm: A numerical method for integrating Newton's equations of motion. It is a time integration algorithm to determine the positions of particles based on their velocities and acceleration.
Heat Transfer: The exchange of thermal energy between objects or systems due to temperature differences, typically through conduction, convection, or radiation.
Internal Energy: The total kinetic energy and potential energy of the particles in a body or system. It's the stored energy within the substance at a particle level.
Diffusion: The net movement of particles from an area of higher concentration to an area of lower concentration. This arises from the random movement and collisions of particles.
Thermodynamics: The study of the relationship involving heat, mechanical work, and other aspects of energy and energy transfer.
States of Matter: The distinct forms that matter can take, traditionally solid, liquid, and gas, which differ in properties such as compressibility, shape, and volume.
Kinetic Energy: The energy that an object possesses due to its motion. In the kinetic model, this refers to the energy of individual particles.
Potential Energy: The energy stored in a system, such as due to the arrangement of particles or interactions with other particles. In the kinetic model, potential energy refers to the energy stored in the interactions between particles.
Description
This model is constructed using the Lennard-Jones potential truncated at a distance of 3 molecular diameters. The motion of the molecules is governed by Newton's laws, approximated using the Verlet algorithm with the indicated Time step. For sufficiently small time steps dt, the system's total energy should be approximately conserved.
Big ideas
The big ideas that help to organize and connect the concepts and content in this section are:
1. When a substance gains or loses heat, the substance may change its temperature, change its state, change its volume or change to a different substance. The kinetic model allows us to understand the macroscopic properties of matter and changes in its state in terms of its microscopic molecular structure and behaviour.
2. Energy may be transferred through all materials and through free space (vacuum). Our understanding of the different mechanisms of heat transfer through different materials enables us to control and make use of heat transfer in many appliances and machines.
3. Internal energy of a body consists of the total kinetic energy and potential energy of the particles in the body. Changes in a body or a substance due to heat gain or heat loss may be explained by the change in its internal energy.
4. Thermodynamics is the study of the relationship involving heat, mechanical work and other aspects of energy and energy transfer. The first law of thermodynamics is a general statement of the law of conservation of energy that includes energy transfer through heat as well as mechanical work. The ideal gas equation gives the relationship of the pressure, volume, temperature and number of moles of a gas. This equation allows us to find the state of a gas in any situation.
Key inquiry question:
How can we explain the effects of heat gain or heat loss on matter?
1. States of matter
• The physical properties of solids, liquids and gases and their molecular structures may be related to the forces and distances between molecules and to the motion of the molecules.
3. & 4. Expansion and contraction, and Kinetic model of matter
• When the temperature of matter (solid, liquid or gas) increases, thermal energy is transferred to the molecules and the molecules gain kinetic energy, causing them to move faster.
• The pressure exerted by a gas may be related to the collisions of the gas molecules with the walls of the container, causing a force per unit area (i.e. pressure) acting on the container. Using the kinetic model, we can explain the relationships of the pressure, volume and temperature of a gas.
Students’ prior knowledge of Kinetic Model of Matter
Primary level:
Students will have learnt that:
• there are three states of matter - solid, liquid and gas. The states of matter may be defined by their physical properties.
• water can exist in the solid, liquid and gaseous states. A change of state can occur when water gains or loses heat.
• solids, liquids and gases can expand when they are heated (gain heat) and contract when they are cooled (lose heat).
Lower secondary level: Students will have learnt that:
• matter is made up of small discrete particles which are in constant and random motion (kinetic theory of matter). The space between particles, their arrangement and movement determine the characteristics of the three states of matter (solid, liquid and gas).
• models (like particulate model) are constructed, justified and continuously revised as new evidence is gathered to explain a phenomena (e.g. properties of solids, liquids and gases, and changes of state such as melting and boiling).
• generally, solids, liquids and gases expand when heat is absorbed and contract when heat is given out. A notable exception is water.
• effects and applications of expansion and contraction occur in everyday life.
Students’ common misconceptions and learning difficulties in Kinetic Model of Matter
Heat gain/loss: Students have difficulty understanding that heat is energy in transit. If heat transfers to or from a substance, the substance may change its temperature, its state, its volume or change to a different substance.
Students often think that:
- heat and cold are kinds of substances that flow into and out of materials, when in fact, only heat is transfer as a result of collision between high energy particles to low high particles.
- objects can have a certain quantity of heat in them, when in fact the concept of internal energy ( stored potential energy U) is different from heat transfer, Q.
- individual particles move in random motion, when in fact they move in constant direction and speed until a collision with another particle or wall, the random motion is the pollen in Brownian motion.
- particles seek to move down a concentration gradient during diffusion, when in fact each particle move in constant direction and speed until a collision with another particle or wall. It is actually the system of collisions that causes the diffusion to spread through higher concentration to lower concentration.
- particulate motion stops when the particles are uniformly distributed in the fluid mixture, when in fact they still continue to move about just that the sum of the system of particle is in dynamic equilibrium.
Other Additional resources
- http://physics.weber.edu/schroeder/md/ Amazing html5 applet
- /http://www.harcourtschool.com/activity/states_of_matter/molecules.swf
- http://netlogoweb.org/launch#http://netlogoweb.org/assets/modelslib/Sample%20Models/Chemistry%20&%20Physics/GasLab/GasLab%20Gas%20in%20a%20Box.nlogo
- http://netlogoweb.org/launch#http://netlogoweb.org/assets/modelslib/Sample%20Models/Chemistry%20&%20Physics/GasLab/GasLab%20Two%20Gas.nlogo
- https://wordwall.net/play/31034/362/222 maze game
- https://wordwall.net/embed/play/31036/019/838 matching game
Explore how molecules in a gas move relative to each other.
Molecules are in constant motion. "Mark two atoms," then run the model to see how the molecules move relative to each other. How do the distances between gas molecules change over time?
http://lab.concord.org/embeddable.html#interactives/sam/phase-change/2-two-types-of-gases.json
Explore how molecules in a liquid move.
Molecules are in constant motion. "Mark two atoms," then run the model to see how the molecules in a liquid move relative to each other. How does the movement of molecules explain why liquids take the shape of their containers?
http://lab.concord.org/embeddable.html#interactives/sam/phase-change/3-liquids.json
What does a solid look like at the molecular level?
Molecules are in constant motion—even those in a solid! "Mark two atoms," then run the model to see how the molecules in a solid move relative to each other. How would you describe the movement and arrangement of molecules in a solid?
http://lab.concord.org/embeddable.html#interactives/sam/phase-change/4-solids.json
Explore how states of matter are related to the strength of intermolecular attractions.
There are three states of matter—solid, liquid and gas. Run the model and change the strength of attractions between the molecules. How does changing the force of attraction between molecules affect the state of that material?
Explore how energy input causes matter to change states.
Matter exists as solids, liquids and gases, and can change state between these.
The model shows a liquid material on the left (small atoms). The amount of heat energy is shown by kinetic energy (KE) shading, with deeper shades of red representing more energetic atoms. On the right side of the barrier is a solid material (large atoms).
Run the model. How much energy is able to penetrate the barrier? Remove the barrier. How quickly do the more energetic atoms melt the solid?
FAQ: Understanding Matter, Energy, and Simulations
- What is the main purpose of the Molecular Dynamics JavaScript Performance Model, and how does it work?
- The primary purpose of this model is to test the speed and performance of JavaScript for computationally intensive simulations, specifically the movement of particles governed by the Lennard-Jones force. The model calculates the trajectory of these particles and allows users to modify parameters such as the number of particles, frames per second, and the number of Verlet steps (a numerical method for solving differential equations) between frames. It simulates the motion of molecules, offering a visual representation of their interactions.
- What are the core "big ideas" that the educational resources are trying to convey related to heat and energy?
- The resources highlight several key concepts, including the idea that heat can cause substances to change temperature, state, or volume. Energy can transfer through materials and space, and the internal energy of a body consists of the kinetic and potential energy of its constituent particles. Finally, thermodynamics explores the relationship between heat, work, and other forms of energy transfer, and the ideal gas equation helps to define the state of a gas.
- How does the kinetic model explain the properties of solids, liquids, and gases?
- The kinetic model explains that matter is made up of constantly moving particles. The arrangement, movement, and space between these particles differ in solids, liquids, and gases and thus these differences result in the distinct physical properties of each state. For example, in solids, particles are tightly packed and vibrate in fixed positions, while in liquids, they have more freedom to move and can flow. In gases, particles move freely and are widely separated.
- What happens to molecules when a substance gains or loses heat according to the kinetic model?
- According to the kinetic model, when a substance gains heat, the molecules gain kinetic energy and move faster. This increased motion can lead to expansion. Conversely, when a substance loses heat, the molecules lose kinetic energy, move slower, and may contract. It is important to note that the state (solid, liquid or gas) of a substance can also change as energy is added or removed.
- What are some common misconceptions that students have about heat and the movement of particles, and how does this resource address them?
- The resource points out several misconceptions, including the idea that "heat" and "cold" are substances and not energy. Students often think that objects contain heat, rather than having internal energy. They also often misunderstand the random nature of particle motion, which is actually a result of constant collisions with each particle moving in a straight line until it collides with something else. Similarly, they misinterpret diffusion as particles seeking to move down a concentration gradient rather than a system of collisions. The resources use simulations to demonstrate correct particle behavior to help dispel these misconceptions.
- How do the provided interactive models demonstrate the movement of molecules in different states of matter?
- The provided links offer interactive simulations where users can observe the behavior of molecules in various states. For example, one model shows the constant motion of molecules in gases and how their distances change over time. Another allows the visualization of molecules in a liquid and demonstrates why it takes the shape of its container. The solid model shows molecules vibrating in place. There's even a model showing the effect of changing the intermolecular forces on the state of matter and the energy required for phase changes between the different states.
- What is the significance of the Verlet algorithm and the "dt" parameter in the Molecular Dynamics Simulation?
- The Verlet algorithm is a numerical method used to approximate the motion of particles by solving the equations of motion. The "dt" parameter represents the time step used in the calculation. For the simulation to be accurate, the time step must be sufficiently small so as to maintain energy conservation. If "dt" is too large, the simulation becomes unstable, and the model needs to be reset to a smaller time step.
- Beyond the specific simulations of matter, what other types of educational models and resources are available on the platform?
- The platform offers a wide array of interactive resources and simulations covering various topics, including but not limited to, mechanics (such as inclined planes, projectiles, and circular motion), electromagnetism (generators, motors, magnetic fields), optics (ray diagrams, diffraction), thermodynamics, nuclear physics (radioactive decay), wave phenomena (interference, superposition), data analysis and statistics (data fitting, distribution of means), and even primary school math and science concepts. There are also numerous simulations designed to be used with video analysis tools like Tracker.
- Details
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 14443