About
MODERN PHYSICS Quantum Physics The photoelectric effect
Description
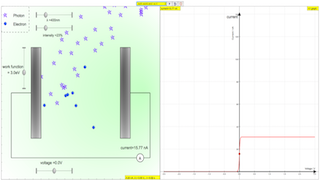
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner can be called photoelectrons. The phenomenon is commonly studied in electronic physics, as well as in fields of chemistry, such as quantum chemistry or electrochemistry.
Sample Learning Goals
- (a) show an appreciation of the particulate nature of electromagnetic radiation
- (b) recall and use the equation E = hf
- (c) show an understanding that the photoelectric effect provides evidence for the particulate nature of electromagnetic radiation while phenomena such as interference and diffraction provide evidence for the wave nature
- (d) recall the significance of threshold frequency
- (e) recall and use the equation 0.5 mvmax 2 = eVs , where Vs is the stopping potential
- (f) explain photoelectric phenomena in terms of photon energy and work function energy
- (g) explain why the stopping potential is independent of intensity whereas the photoelectric current is proportional to intensity at constant frequency (h) recall, use and explain the significance of the equation hf = Φ + 2 1 mvmax 2
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits



Fu-Kwun Hwang; lookang; tina; Flix J. Garca Clemente
Briefing Document: Photoelectric Effect Simulation
1. Overview:
This document reviews a web-based interactive simulation of the photoelectric effect, hosted by Open Educational Resources / Open Source Physics @ Singapore. The simulation is designed as a learning tool for physics, particularly within the context of quantum physics and modern physics concepts. It aims to help students understand the fundamental principles of the photoelectric effect and the particle nature of light.
2. Core Concepts and Learning Goals:
The simulation is structured to facilitate understanding of the following key concepts:
- Particulate Nature of Electromagnetic Radiation: The simulation directly addresses the concept that electromagnetic radiation, such as light, is composed of discrete particles called photons. This aligns with the learning goal to "show an appreciation of the particulate nature of electromagnetic radiation."
- Energy of a Photon: The learning goal to "(b) recall and use the equation E = hf" emphasizes understanding that the energy of a photon (E) is directly proportional to its frequency (f) through Planck's constant (h).
- Wave-Particle Duality: The simulation aims to contrast the particulate nature of light as demonstrated by the photoelectric effect with wave-like phenomena such as interference and diffraction. As stated in the source, the simulation is intended to help learners "show an understanding that the photoelectric effect provides evidence for the particulate nature of electromagnetic radiation while phenomena such as interference and diffraction provide evidence for the wave nature".
- Threshold Frequency: The simulation is intended to help users understand the idea that "recall the significance of threshold frequency". This is the minimum frequency of light required to eject electrons from a metal surface.
- Stopping Potential: The simulation emphasizes understanding "the equation 0.5 mvmax 2 = eVs , where Vs is the stopping potential" , which relates the maximum kinetic energy of ejected electrons to the stopping potential required to halt the photocurrent.
- Work Function: The simulation is geared towards helping users "explain photoelectric phenomena in terms of photon energy and work function energy". The work function (Φ) is the minimum energy required to eject an electron from a particular metal.
- Intensity and Photoelectric Current: Users of the simulation should be able to "explain why the stopping potential is independent of intensity whereas the photoelectric current is proportional to intensity at constant frequency". This refers to the understanding that while the intensity affects the number of photoelectrons emitted, the energy of each photoelectron (and thus stopping potential) only depends on the frequency of the light.
- Key Photoelectric Effect Equation: The simulation supports learning the equation " hf = Φ + 2 1 mvmax 2", with the learning goal to "recall, use and explain the significance of the equation". This equation mathematically expresses the conservation of energy during the photoelectric effect.
3. Simulation Features:
- Interactive: The applet is described as an "JavaScript HTML5 Applet Simulation Model" meant to be used directly in a web browser, making it easily accessible on various platforms. The fact that it is explicitly designed to work on "Android/iOS including handphones/Tablets/iPads" and "Windows/MacOSX/Linux including Laptops/Desktops" underscores its broad usability.
- Embeddable: The fact that the simulation can be "Embed this model in a webpage:" through an iframe suggests its ease of use in different online educational contexts.
- Multiple Versions: The simulation seems to have multiple versions or iterations available as described by the "Version" list, suggesting an evolution of its design and functionality.
4. Target Audience:
The material is explicitly described as suitable for "Junior College" students, which corresponds to high school level education, though could also be used in more introductory college courses. The simulation is designed for students learning about "MODERN PHYSICS Quantum Physics The photoelectric effect".
5. Additional Resources:
The document lists a range of additional resources that can support learning of the photoelectric effect:
- External links to other simulations: The "Other resources" section provides links to different implementations of the photoelectric effect simulation, allowing users to explore various visual and interactive models.
- Video Tutorials: The "Video" section points to YouTube tutorials of the photoelectric effect. This supports diverse learning styles by providing complementary instruction.
- Blog post by Loo Kang Wee: Indicates there is additional discussion and explanation of the simulation's creation and use.
- Credits and Authors: The document includes the names of the developers, highlighting the collaborative nature of open-source educational resource creation.
6. Platform and Technology:
- EasyJavaScriptSimulation: The simulation uses the "EasyJavaScriptSimulation" framework, which indicates a technology-specific approach to its development.
7. Importance of Simulation:
The simulation aims to address common learning difficulties in understanding quantum physics, particularly with the counter-intuitive nature of the wave-particle duality. By presenting an interactive visual representation of the photoelectric effect, the model enables students to engage with abstract concepts and develop a more concrete grasp of the phenomena. It is also part of a large suite of interactive simulations created by the Open Source Physics @ Singapore initiative, which indicates its support for active and visual learning techniques.
8. Open Educational Resources:
- The document highlights that the materials are "licensed Creative Commons Attribution-Share Alike 4.0 Singapore License," showing a commitment to freely accessible education. The fact that is is part of an "Open Educational Resources" initiative indicates the materials support wide adoption and modification of the tools for educational use.
In conclusion:
This web-based interactive simulation of the photoelectric effect is a valuable educational resource. It is designed to support a solid understanding of the key principles of the photoelectric effect, the particle nature of light, and the critical relationships between photon energy, work function, and stopping potential. The use of the "EasyJavaScriptSimulation" framework allows for easy web-based access, and the open-source nature of the simulation facilitates broad distribution and adaptation.
Photoelectric Effect Study Guide
Quiz
Instructions: Answer the following questions in 2-3 sentences each.
- What is the photoelectric effect?
- What are photoelectrons?
- What does the equation E = hf represent in the context of the photoelectric effect?
- Explain the significance of the threshold frequency in the photoelectric effect.
- What is the stopping potential (Vs)?
- What is the relationship between photon energy and work function energy in the photoelectric effect?
- Why is the stopping potential independent of light intensity?
- How is the photoelectric current related to light intensity at a constant frequency?
- Explain how the photoelectric effect provides evidence for the particulate nature of electromagnetic radiation.
- How does the phenomenon of interference and diffraction provide evidence of the wave nature of light?
Quiz Answer Key
- The photoelectric effect is the phenomenon where electrons are emitted from a material, typically a metal, when light shines on it. This effect demonstrates that light can interact with matter as discrete packets of energy.
- Photoelectrons are the electrons that are ejected from a material's surface due to the photoelectric effect. These electrons gain kinetic energy from the incident light.
- The equation E = hf represents the energy of a photon, where E is the energy, h is Planck's constant, and f is the frequency of the electromagnetic radiation. This illustrates how light energy is quantized.
- The threshold frequency is the minimum frequency of light required to eject electrons from a material via the photoelectric effect. If the light's frequency is below this threshold, no electrons will be emitted, no matter how intense the light is.
- The stopping potential (Vs) is the voltage required to stop the most energetic photoelectrons from reaching the collector plate in a photoelectric effect experiment. It is a measure of the maximum kinetic energy of the photoelectrons.
- In the photoelectric effect, photon energy (hf) must be greater than or equal to the material's work function (Φ) for electrons to be emitted. The difference between photon energy and work function energy becomes the kinetic energy of the emitted electrons.
- The stopping potential is independent of light intensity because the energy of the individual photons is determined solely by their frequency. While intensity impacts the number of photoelectrons, it doesn't alter their individual maximum kinetic energy.
- At a constant frequency, the photoelectric current is directly proportional to the light's intensity. Higher intensity means more photons and, therefore, more emitted photoelectrons per second.
- The photoelectric effect supports the particulate nature of electromagnetic radiation because it shows that light energy comes in discrete packets (photons). These photons can interact with individual electrons, which could not be explained through wave theory alone.
- Phenomena such as interference and diffraction demonstrate the wave-like nature of light as they can only be explained with wave interactions like constructive and destructive interference.
Essay Questions
Instructions: Answer the following questions in essay format, drawing upon your understanding of the provided source material and your knowledge of physics.
- Discuss how the photoelectric effect revolutionized our understanding of light and the nature of electromagnetic radiation. How did it challenge classical wave theory?
- Explain the experimental setup of a photoelectric effect experiment and how it is used to determine the stopping potential and work function of a material.
- Compare and contrast the evidence for the wave-like and particle-like behavior of light. How do both phenomena contribute to our overall understanding of light?
- Analyze the equation hf = Φ + ½mvmax² and explain the significance of each term, including how the equation relates the particle-like nature of light to electron behavior.
- Explore the various applications of the photoelectric effect in technology and scientific fields. Provide examples of how the principles of the photoelectric effect are applied in practical scenarios.
Glossary of Key Terms
- Photoelectric Effect: The phenomenon where electrons are emitted from a material, typically a metal, when light shines upon it.
- Photoelectrons: Electrons that are ejected from a material's surface due to the photoelectric effect.
- Photon: A discrete packet of electromagnetic radiation or light, carrying a specific amount of energy.
- Planck's Constant (h): A fundamental physical constant that relates the energy of a photon to its frequency; approximately 6.626 x 10^-34 joule-seconds.
- Energy of a Photon (E=hf): The energy of a single photon, where E is the energy, h is Planck's constant, and f is the frequency of the electromagnetic radiation.
- Threshold Frequency (f0): The minimum frequency of light required to eject electrons from a material via the photoelectric effect.
- Work Function (Φ): The minimum energy required to remove an electron from the surface of a material.
- Stopping Potential (Vs): The voltage required to stop the most energetic photoelectrons from reaching the collector plate in a photoelectric effect experiment; a measure of the maximum kinetic energy of the photoelectrons.
- Electromagnetic Radiation: Energy in the form of waves or particles, having both electrical and magnetic field components; includes light, radio waves, and x-rays.
- Quantum Mechanics: A fundamental theory in physics that describes the physical properties of nature at the scale of atoms and subatomic particles, which includes the quantization of energy.
- Interference: The phenomenon when two or more waves overlap in space resulting in a combined wave.
- Diffraction: The bending of waves around obstacles or through apertures.
https://play.google.com/store/apps/details?id=com.ionicframework.photoelectricapp781205&hl=en

https://itunes.apple.com/us/app/photoelectric-effect-simulator/id1181806293?ls=1&mt=8
Version:
- http://iwant2study.org/lookangejss/06QuantumPhysics/ejss_model_photoelectriceffectwee1/photoelectriceffectwee_Simulation.xhtml
- http://iwant2study.org/lookangejss/06QuantumPhysics/ejss_model_photoelectriceffectwee1/photoelectriceffectwee1_Simulation.xhtml
- http://weelookang.blogspot.sg/2016/01/ejss-photoelectric-effect-model.html Blogpost by Loo Kang Wee
- ejs_users-ntnu-fkh-photoelectriceffect.jar mirror copy
- http://www.phy.ntnu.edu.tw/ntnujava/index.php?topic=1655.0 by Fu-Kwun Hwang
Other resources
http://www.kcvs.ca/site/projects/JS_files/Photoelectric_Effect/PhotoElectric.html
Video
I would love to emded video tutorials made using my OSP simulation! let me know :)
- Photoelectric Basics by Frank McCulley
- Photoelectric Effect Demonstration by National STEM Centre
- A Level Physics - The Photoelectric Effect by A Level Physics Online
Frequently Asked Questions about the Photoelectric Effect
- What is the photoelectric effect?
- The photoelectric effect is a phenomenon where electrons are emitted from a material, typically a metal, when light shines on it. These emitted electrons are called photoelectrons. This effect is a core concept in both physics and chemistry, particularly in areas such as quantum physics and electrochemistry. It provides crucial evidence that light can behave as a particle, not just as a wave.
- How does the photoelectric effect demonstrate the particulate nature of electromagnetic radiation?
The photoelectric effect shows that light energy is delivered in discrete packets called photons. When a photon interacts with an electron in a material, it transfers its energy to that electron. If this energy is sufficient, the electron is emitted. This particle-like interaction contrasts with classical wave theory, which would predict that any intensity of light should be able to eject electrons, given enough time.
- What is the significance of the threshold frequency in the photoelectric effect?
The threshold frequency is the minimum frequency of light required to eject electrons from a particular metal. If the light's frequency is below this threshold, no electrons will be emitted, no matter how intense the light is. This observation is key evidence for the quantum nature of light, as it implies that each photon carries a specific amount of energy determined by its frequency, and only photons with sufficient energy (determined by the threshold frequency) can eject electrons.
- What is the relationship between photon energy, work function, and kinetic energy of emitted photoelectrons?
The energy of a photon (E) is given by the equation E = hf, where h is Planck's constant and f is the frequency of the light. When a photon hits a metal surface, some of this energy (Φ) is used to overcome the work function of the metal, which is the minimum energy needed to release an electron. The remaining energy becomes the kinetic energy of the emitted photoelectron. This is expressed by the equation hf = Φ + 1/2 mvmax², where 1/2 mvmax² is the maximum kinetic energy of the emitted photoelectrons.
- What does the equation E = hf represent?
- The equation E = hf relates the energy of a photon (E) to its frequency (f), where 'h' is Planck's constant. This equation is fundamental to quantum mechanics, showing that light's energy is quantized, meaning it comes in discrete units of energy called photons, with each photon's energy being directly proportional to its frequency.
- What is the stopping potential and how is it related to the kinetic energy of photoelectrons?
- The stopping potential (Vs) is the voltage needed to completely stop the emitted photoelectrons. When electrons are emitted with kinetic energy they can, and will move to areas of lower potential. A positive potential applied in the direction of motion of the electron will counteract it's momentum and slow it down. If this potential is large enough the electron will not have enough kinetic energy to reach the anode and that potential is called the stopping potential. The maximum kinetic energy of the photoelectrons (0.5 mvmax²) is equal to the product of the electron's charge (e) and the stopping potential (Vs). Thus, 0.5 mvmax² = eVs. This relationship means that the stopping potential directly measures the maximum kinetic energy of the photoelectrons.
- Why is the stopping potential independent of light intensity, whereas the photoelectric current is proportional to intensity at constant frequency?
- The stopping potential is determined by the maximum kinetic energy of the photoelectrons, which, in turn, depends on the frequency (and energy) of individual photons. Increasing light intensity means more photons are hitting the surface, so you have more electrons are ejected which increases the current, but it does not increase the energy of each individual photon, and thus doesn't effect the energy of the electron. Therefore the stopping potential remains unchanged. On the other hand, the current is determined by the number of photoelectrons emitted per second. Higher light intensity means more photons, and thus more electrons, are emitted, resulting in a higher photoelectric current, given that the light has a frequency above the threshold.
- What is the significance of the photoelectric effect in demonstrating wave-particle duality?
- The photoelectric effect demonstrates the particle-like nature of light. The energy of a light beam is carried by a flux of photons. In contrast, phenomena like interference and diffraction demonstrate the wave-like nature of light, where waves can superimpose or bend around obstacles. Together, these effects provide evidence that light exhibits a dual nature, behaving as both particles and waves, depending on the experimental context. This wave-particle duality is a fundamental concept in quantum mechanics.
- Details
- Written by Loo Kang Wee
- Parent Category: Physics
- Category: 06 Modern Physics
- Hits: 39974