https://iwant2study.org/moodle402/mod/laejss/view.php?id=34
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits

Zenia Chng; Lawrence Wee
Launch URL https://iwant2study.org/moodle402/enrol/lti/launch.php
Custom properties id=6ddc0320-d752-46b7-b771-10b3b8808024
Together https://iwant2study.org/moodle402/enrol/lti/launch.php?id=6ddc0320-d752-46b7-b771-10b3b8808024
Objectives
- Identify in writing, the different types of of elements and number of atoms given a chemical formula and Periodic Table.
- By writing the numerical coefficients, balance chemical equations, recognising that the number of atoms of the reactants must be equal to the number of atoms of the products.
Brief Description
Guide for teachers using the simulation
- There are 3 different levels of increasing difficulty. (Level 1 - 3 questions, Level 2 - 4 questions, Level 3 - 3 questions).
- The "hint" button is only enabled after students have made an attempt on the question (i.e. after clicking on "check" once). Upon answering a question correct, the next question will be shown. Students are able to toggle back and forth the questions they have attempted, but will not be able to proceed if they have not cleared the current question.
- When students click "hint", reactant(s)/product(s) particle(s) that has/have the incorrect stoichiometric coefficient will be highlighted in red.
- particles highlighted in solid red lines - excess number of particles
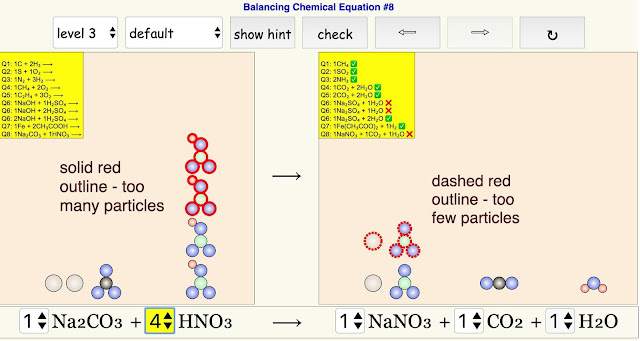
- particles highlighted in dashed red lines - insufficient number of particles
- particles highlighted in solid red lines - excess number of particles
- There are 3 different views available for students - default, show chemical symbol and show charges
- The default view shows the pictorial representation of particles, available for all 3 levels.
- "Show chemical symbol" is also available for all 3 levels, but it shows the chemical symbol of each particle (including charges of ionic compounds).
- "Show charges" is only applicable to questions with ionic compounds (i.e. question 6 onwards). Its representation of ions is similar to that of 'dot-and-cross' diagrams.
- For monitoring of student's progress, there are two yellow boxes found on the top left hand corner of the reactant and product boxes. It shows the log of student's answers.
- If an equation is balanced correctly, a green tick will appear on the side of the products panel.
- If an equation is balanced incorrectly, a red cross will appear on the side of the products panel.
- If a student click on the "refresh" button in the simulation, the log will not reset. However, if the student was to refresh the browser, the log will automatically be refreshed as well.
Additional notes
-
9 questions of different levels of difficulty (level 1, level 2 and level 3) with increasing number of products on the right hand side of the equal sign
- show hint button, temporary reveals the shortage (dash outlines) or extra (solid outline) atoms
- check button, evaluate the students' choice on the bottom combobox numbers, when correct, the next question will be fielded
- left arrow button, to allow students to revisit questions they got correct
- right arrow button, to allow students to progress only after they got the questions correct, but not before
- visualisation panels of up to 6 molecules on the left (reactants) and right (products) of the reaction
- simple data analytics of the students' actions, that stays on screen even after reset button, to help teachers' give evidence based feedback to improve students' understanding.
Guide for students
In the simulation, there are 10 questions of increasing difficulty. After you have balanced the chemical equation, you will be shown the next question. You will only be allowed to toggle between levels and questions after you have answered them correctly for the first time.
The “hint” button will only be active after you have attempted the question once. To reveal the hint, click and hold on the button.
If you see a solid red outline around the particle, it would mean there are too many particles for that reactant/product. A dashed red outline refers to too few particles.
There are three different pictorial representation for you to choose from – default view, particles with chemical symbols, and particles represented as ions.
Question: Sulfur dioxide, SO2 can pollute the air and causes health problems. Which of the following clearly shows the number of atoms of sulfur and oxygen present in three molecules of sulfur dioxide?
3 sulfur atoms and 6 oxygen atoms
Propane \( C_{3}H_{8} \), undergoes complete combustion in excess oxygen. Which of the following is the correct balanced chemical equation for the complete combustion of propane?
\( C_{3}H_{8} + 5O_{2} -> 3CO_{2} + 4H_{2}O\)
Video
[text]
Version:
- https://weelookang.blogspot.com/2019/11/balancing-chemistry-equation-javascript.html
- https://vle.learning.moe.edu.sg/community-gallery/lesson/view/c2cd909f-5ed7-49f3-9959-3ac4bcf6a71f SLS lesson
Other Resources
[text]
end faq
{accordionfaq faqid=accordion4 faqclass="lightnessfaq defaulticon headerbackground headerborder contentbackground contentborder round5"}
- Details
- Written by Loo Kang Wee
- Parent Category: Chemistry
- Category: 03 Chemistry of Reactions
- Hits: 9211


.png
)




