About
This will be a EJSS-based particle physics simulation
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Credits

rytan451; lookang
This briefing document summarizes the key themes and ideas presented in the provided sources regarding the factors affecting reaction rates. The sources highlight an interactive simulation designed to visually demonstrate these principles.
Source 1: "Rates of Reactions with Concentration of Reactant 1 (Blue), Temperature and Amount and Surface Area of (Catalyst -Yellow)" by rytan451 and lookang.
This source serves as the title and authorship attribution for the simulation itself. It explicitly states the key factors being explored:
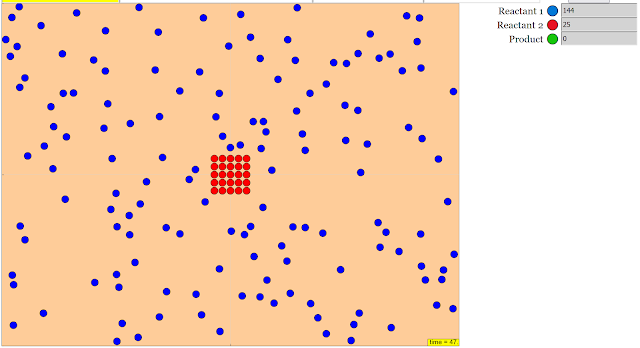
- Concentration of Reactant 1 (Blue): This indicates that the simulation allows for manipulation of the initial amount of one specific reactant and uses a color (blue) to represent it within the simulation.
- Temperature: The simulation incorporates the effect of temperature on reaction rates.
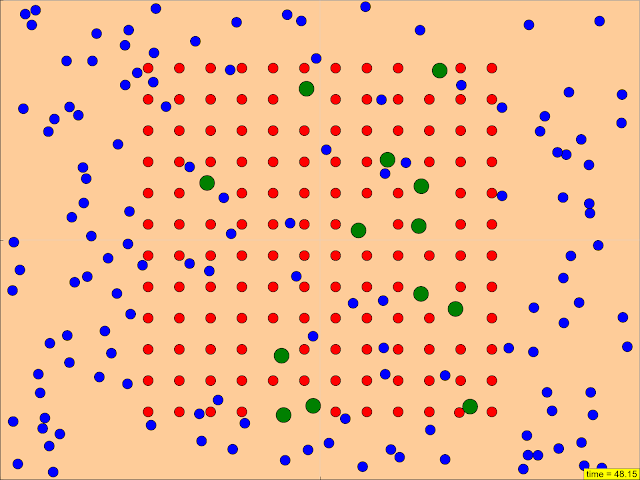
- Amount and Surface Area of Catalyst (Yellow): This highlights the role of a catalyst, specifically allowing for changes in both the quantity and surface area of the catalyst, represented by the color yellow.
Source 2: "Rates of Reactions with Concentration of Reactant 1 (Blue), Temperature and Amount and Surface Area of Catalyst (Yellow) JavaScript Simulation Applet HTML5 - Open Educational Resources / Open Source Physics @ Singapore"
This source provides context for the simulation, identifying it as an "Open Educational Resources / Open Source Physics @ Singapore" project. It details the nature and purpose of the simulation:
- Interactive Simulation: The core nature of the resource is an interactive simulation, allowing users to actively engage with the concepts.
- Educational Demonstration: The primary purpose is to serve as an "educational demonstration of factors affecting the rate of reaction."
- Realistic Physics Model: The simulation utilizes a "realistic physics model" to depict particle interactions and their influence on reaction speed.
- Configurable Conditions: Users can "Configure starting and boundary conditions," suggesting control over the initial parameters of the reaction.
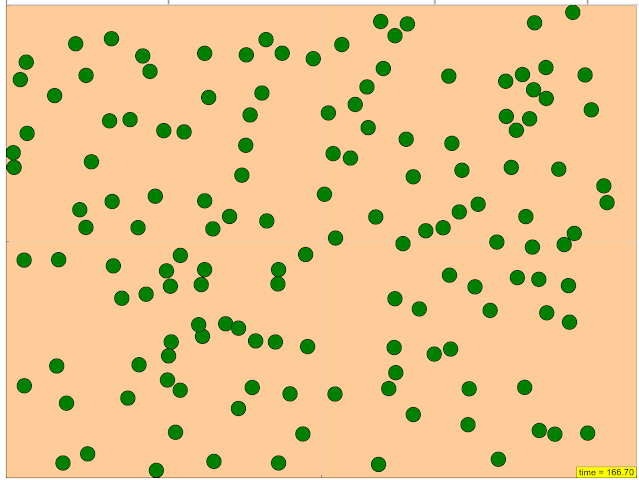
- Visual Observation: The simulation allows users to "Watch the particles interact in real time" and "See how many of each type of particle there are," providing a visual representation of the reaction process and the changing quantities of reactants and products.
- Manipulation of Surface Area: The ability to "Change the surface area of a reactant" is explicitly mentioned as a feature, reinforcing the importance of this factor.
- Keywords: Relevant keywords associated with the simulation include "Interactive Simulation," "Chemistry," and "Reaction," confirming its focus on chemical kinetics.
- Target Audience: The simulation is explicitly labelled "For Teachers," with sample learning goals and research sections (though the text for these is not provided), suggesting its intended use in an educational setting.
Main Themes and Important Ideas/Facts:
Based on the provided sources, the main themes and most important ideas are:
- Factors Affecting Reaction Rates: The simulation focuses on demonstrating the impact of three key factors on the rate of a chemical reaction:
- Concentration of Reactants: Higher concentrations generally lead to more frequent collisions between reactant particles, thus increasing the reaction rate.
- Temperature: Increased temperature provides particles with more kinetic energy, resulting in more frequent and energetic collisions, and a higher proportion of successful collisions (those that lead to a reaction).
- Catalyst (Amount and Surface Area): A catalyst speeds up a reaction without being consumed. The simulation likely demonstrates that increasing the amount or surface area of the catalyst provides more active sites for the reaction to occur, thereby increasing the reaction rate.
- Interactive Learning: The simulation is designed as an interactive tool to facilitate learning about reaction rates. The ability to manipulate variables and observe the real-time effects is a key pedagogical approach.
- Visual Representation: The simulation utilizes a "realistic physics model" and allows users to "Watch the particles interact in real time," emphasizing the visual nature of understanding reaction kinetics at the particle level.
- Open Educational Resource: The simulation is freely available and intended for educational use, aligning with the principles of Open Educational Resources.
Key Facts Extracted:
- The simulation focuses on "Rates of Reactions with Concentration of Reactant 1 (Blue), Temperature and Amount and Surface Area of (Catalyst -Yellow)."
- It is a "JavaScript Simulation Applet HTML5" developed by rytan451 and lookang.
- It is an "educational demonstration" using a "realistic physics model."
- Users can "Configure starting and boundary conditions," "Watch the particles interact in real time," "See how many of each type of particle there are," and "Change the surface area of a reactant."
- The simulation is available as an "Open Educational Resources / Open Source Physics @ Singapore."
In conclusion, these sources describe an interactive educational simulation designed to visually demonstrate how changing the concentration of a reactant, the temperature, and the amount and surface area of a catalyst influence the rate of a chemical reaction. It is intended as a tool for teachers to help students understand these fundamental concepts in chemistry.
Reaction Rate Study Guide
Quiz
- What three factors are explicitly mentioned in the simulation title as affecting the rate of reaction?
- What is the role of the "Blue" particles in the simulation?
- What is the role of the "Yellow" particles in the simulation?
- How does increasing the concentration of the "Blue" reactant typically affect the rate of reaction?
- How does changing the temperature generally influence the rate of a reaction?
- Besides the amount of catalyst, what other characteristic of the catalyst is highlighted as affecting the reaction rate?
- What type of simulation is this described as in the "About" section?
- Where can teachers find the simulation to use for educational demonstrations?
- The simulation uses a "realistic physics model." What does this suggest about how the reaction is represented?
- What does the simulation allow users to configure in terms of starting and boundary conditions?
Essay Questions
- Discuss the interplay between temperature and the concentration of reactant 1 (Blue) on the overall rate of reaction as depicted in the simulation. How might these factors interact to influence the frequency of effective collisions?
- Analyze the role of the catalyst (Yellow) in the simulation. Explain how both the amount and surface area of the catalyst contribute to altering the reaction rate.
- Describe how the simulation could be used as an educational tool to demonstrate the concepts of collision theory in relation to the factors affecting reaction rates. Provide specific examples of how adjusting parameters in the simulation would illustrate these principles.
- Based on the information provided, propose an experiment that could be designed using this simulation to investigate the quantitative relationship between one of the listed factors (concentration, temperature, or catalyst characteristics) and the reaction rate. Outline the steps of the experiment and the expected observations.
- Evaluate the strengths and limitations of using a simulation like this to teach students about reaction rates compared to conducting a physical laboratory experiment.
Glossary of Key Terms
- Rate of Reaction: A measure of how quickly reactants are consumed or products are formed in a chemical reaction.
- Concentration: The amount of a substance in a given volume. In the context of the simulation, likely refers to the number of reactant particles.
- Temperature: A measure of the average kinetic energy of the particles in a substance. Higher temperature means faster-moving particles.
- Catalyst: A substance that increases the rate of a chemical reaction without being consumed in the process.
- Surface Area: The total area of the exposed surface of a substance. For a solid catalyst, a larger surface area means more sites for the reaction to occur.
- Reactant: A substance that is consumed during a chemical reaction. In the simulation, the "Blue" particles represent one of the reactants.
- Simulation: A computer-based model that imitates a real-world process or system.
- Particle Physics Simulation: A simulation that models the behavior and interactions of individual particles.
- Educational Demonstration: An activity used to illustrate a concept or principle for teaching purposes.
- Starting Conditions: The initial parameters or settings of a simulation or experiment.
- Boundary Conditions: The conditions that define the limits or constraints of a system in a simulation.
Answer Key
- The three factors are Concentration of Reactant 1 (Blue), Temperature, and Amount and Surface Area of Catalyst (Yellow).
- The "Blue" particles represent Reactant 1 in the simulation.
- The "Yellow" particles represent the Catalyst in the simulation.
- Increasing the concentration of the "Blue" reactant typically increases the rate of reaction because there are more particles available to collide and react.
- Changing the temperature generally influences the rate of a reaction by affecting the kinetic energy of the particles, which influences collision frequency and energy.
- Besides the amount of catalyst, the surface area of the catalyst is highlighted as affecting the reaction rate.
- This is described as an EJSS-based particle physics simulation.
- Teachers can find the simulation on the Open Educational Resources / Open Source Physics @ Singapore website.
- Using a "realistic physics model" suggests that the simulation attempts to mimic the physical interactions and movements of the particles involved in the reaction.
- The simulation allows users to configure starting and boundary condition.
Sample Learning Goals
[text]
For Teachers

Configure starting and boundary conditions
Research
[text]
Video
Version:
Other Resources
[text]
What is the primary purpose of the "Rates of Reactions with Concentration of Reactant 1 (Blue), Temperature and Amount and Surface Area of Catalyst (Yellow)" resource?
The primary purpose of this resource is to provide an educational demonstration of the factors that affect the rate of a chemical reaction. It utilizes an interactive simulation to visualize how different initial conditions influence the speed at which a reaction progresses.
What factors affecting the rate of reaction can be explored using this simulation?
The simulation allows users to explore the impact of several key factors on the rate of reaction:
- Concentration of Reactant 1 (Blue): Changing the amount of the blue reactant.
- Temperature: Adjusting the temperature of the system.
- Amount of Catalyst (Yellow): Varying the quantity of the yellow catalyst.
- Surface Area of Catalyst (Yellow): Altering the surface area of the yellow catalyst.
How does the simulation visually represent a chemical reaction?
The simulation uses a "realistic physics model" where particles representing the reactants and catalyst interact in real-time. Users can observe these particle interactions and see how they lead to the formation of products, allowing for a visual understanding of the reaction process.
What kind of educational tool is this resource?
This resource is an interactive simulation applet built using HTML5 and the Easy JavaScript Simulation (EJSS) toolkit. It is categorized as an Open Educational Resource (OER), meaning it is freely available for educational use.
What are the sample learning goals associated with this simulation?
While the specific text for the sample learning goals is not provided in the excerpt, the resource is designed to help users understand how factors like concentration, temperature, catalyst amount, and catalyst surface area affect the rate of a reaction. It encourages observation of particle interactions and analysis of how starting conditions influence reaction speed.
Who are the creators credited for this resource?
The creators credited for the "Rates of Reactions with Concentration of Reactant 1 (Blue), Temperature and Amount and Surface Area of Catalyst (Yellow)" simulation are rytan451 and lookang.
Where can this simulation be accessed or embedded?
The simulation can be accessed directly via the link provided: https://sg.iwant2study.org/ospsg/index.php/interactive-resources/chemistry/03-chemistry-of-reactions/03-chemical-reactions/1144-rate-of-reaction. It can also be embedded in a webpage using the provided iframe code.
What is the technical foundation of this simulation?
The simulation is based on the Easy JavaScript Simulation (EJSS) toolkit, which is used to create interactive physics simulations. The applet itself is in HTML5, making it accessible across various devices and platforms.
- Details
- Written by Loo Kang Wee
- Parent Category: 03 Chemistry of Reactions
- Category: 03 Chemical Reactions
- Hits: 6768

.png
)






